Abstract
Extracellular vesicles (EVs) are composed of a lipid bilayer containing transmembrane and soluble proteins. Subtypes of EVs include ectosomes (microparticles/microvesicles), exosomes, and apoptotic bodies that can be released by various tissues into biological fluids. EV cargo can modulate physiological and pathological processes in recipient cells through near- and long-distance intercellular communication. Recent studies have shown that origin, amount, and internal cargos (nucleic acids, proteins, and lipids) of EVs are variable under different pathological conditions, including cardiovascular diseases (CVD). The early detection and management of CVD reduce premature morbidity and mortality. Circulating EVs have attracted great interest as a potential biomarker for diagnostics and follow-up of CVD. This review highlights the role of circulating EVs as biomarkers for diagnosis, prognosis, and therapeutic follow-up of CVD, and also for drug delivery. Despite the great potential of EVs as a tool to study the pathophysiology of CVD, further studies are needed to increase the spectrum of EV-associated applications.
Keywords: extracellular vesicles, exosomes, ectosomes, biomarkers, RNA, proteins, lipids, cardiovascular disease
1. Introduction
Extracellular vesicles (EVs) is a generic term for particles naturally released from the cells that are delimited by a lipid bilayer-containing transmembrane and soluble proteins and cannot replicate, according to The International Society for Extracellular Vesicles (ISEV) [1]. The first study that reported EVs was published in 1967 describing EVs as minute dust-like particulate material rich in lipid content [2].
EVs can be classified based on size as small EVs (sEVs), with range < 100 nm or < 200 nm, and medium/large EVs (m/lEVs), with size range > 200 nm [1]. EVs also can be classified based on cell origin as ectosomes (microparticles/microvesicles), exosomes, and apoptotic bodies. Ectosomes (size range 100–500 nm) are released from the plasma membrane budding, exosomes (size range 50–150 nm) are assembled from the endosomal pathway and released by exocytosis of multivesicular bodies (MVB), and apoptotic bodies (size range 500 nm–2 µm) are generated during apoptotic cell shrinkage and death [3,4,5,6]. There are various methods used for isolation of EVs or a specific EV subtype that have been recently reviewed [7], such as ultracentrifugation (UC), size-exclusion chromatography (SEC), filtration, immunoaffinity-based isolation, commercial reagents (using polymers), microfluidics, and asymmetric flow field-flow fractionation (AF4). To increase the specificity or purity, the methods can be combined.
EVs can be characterized by their cargos and surface protein biomarkers, including annexins (e.g., annexin 1, 5, 6, and 11), disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), angiotensin-converting enzyme (ACE), EH domain-containing protein 4 (EHD4), major histocompatibility complex class II (MHC II), flotillin-1 (FLOT1), and heat-shock 70-kDA (HSC70/HSP73, HSP70/HSP72). Other proteins are used as exosome markers, such as tetraspanins (CD9, CD63, CD81, and CD82), stress proteins (Hsc70 and Hsp90), proteins involved in membrane fusion (Rabs, and ARF6), and protein members of the endosomal sorting complex required for transport (Alix and TSG101) [8,9]. Microvesicles have content similar to exosomes that include specific proteins, such as integrins, glycoproteins, and metalloproteinases [8,10]. To identify EV’s protein markers, the main methods include Western blotting, ELISA, flow cytometry (FCM), and nano-FCM. In addition, transmission electron microscopy (TEM), dynamic light scattering (DLS), and nanoparticle tracking analysis (NTA) are commonly used [7,11,12].
EVs have emerged as possible biomarker sources from several diseases, due their ability to modulate near- or in long-distance intercellular communication influencing the disease development and progression [13,14,15]. Intercellular communication consists of transferring EV bioactive cargos or activating signaling pathways to recipient cells, which can lead to phenotypic and functional changes in their target cells [5,16]. EVs are present in various tissues and biological fluids from which they can be recovered and monitored in both physiological and pathological conditions [17,18]. The quantity, origin, and internal cargo (e.g., nucleic acids, proteins, and lipids from parental cells) are variable in different pathophysiological processes [14,19]. EVs also have a metabolically active outer membrane that protects their content until released into recipient cells [17].
Circulating EVs have attracted great interest in the field of cardiovascular medicine due to their high stability. EVs offer a non-invasive access to monitor the status of the cardiovascular diseases (CVD), and the use of circulating EVs as diagnostic biomarkers [13,20]. CVD causes the highest number of deaths and vast health and economic burdens worldwide [21,22]. CVD include several pathologies such as coronary artery disease (CAD), cerebrovascular disease, peripheral arterial disease, ischemic heart disease, hypertension, and heart failure (HF). Early detection and management of CVD can decrease the risk of heart attack and stroke in individuals at high risk of CVD, and, therefore, reduce premature morbidity and mortality [23].
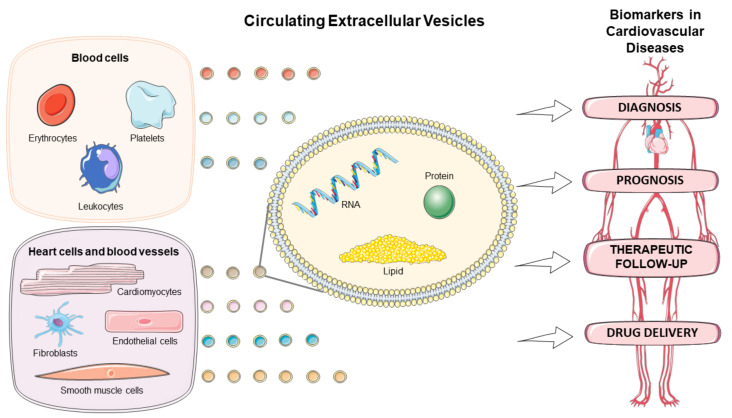
There has been a growing interest in exploring the EVs in the diagnostic, prognostic, and therapeutic monitoring of CVD, as well as drug delivery (Figure 1). This review discusses the role of circulating EVs in CVD based on origin, amount, and content of the EVs, and highlights their application as biomarkers and drug delivery tool in several cardiovascular pathologies.
Figure 1.
Circulating extracellular vesicles as biomarkers for diagnosis, prognosis, therapeutic follow-up, and drug delivery vehicles in cardiovascular diseases. Figure created using Servier Medical Art images (http://smart.servier.com, accessed on 30 December 2020).
2. Origins of Extracellular Vesicles Related to CVD
Circulating EVs are released by almost all cells, including cardiovascular system-related cells (e.g., blood, heart, and blood vessels) [24,25]. Biologic fluids, such as blood [26], urine [27], saliva [28], breast milk [29], and seminal fluid [30], as well as conditioned media from cell culture experiments [31,32], all contain EVs.
2.1. Blood-Cells Derived EVs
EVs can be released from platelets (and megakaryocytes), erythrocytes, and leukocytes. The main sources of circulating EVs are platelets, which are derived from megakaryocytes, and are regulators of hemostasis, inflammation, and vascular integrity [33,34]. Some reviews [35,36,37] have reported the role of platelet-derived EVs in atherosclerosis, acute coronary syndrome (ACS), and thrombosis, being considered as potential EV source in CVD [38]. Platelet EVs have procoagulant and pro-inflammatory effects [39,40,41,42], and serve as important messengers, communicating the changes that occur in the plasma to bone marrow cells [43] and other tissues impermeable to platelets [33].
Circulating EVs derived from erythrocytes are released to clear away harmful molecules and prevent the early removal of these cells from circulation [44,45]. Erythrocyte-derived EVs are also shown to be associated with CVD. Patients with ST-segment elevation myocardial infarction (STEMI) who undergo angioplasty have approximately double of erythrocyte-derived EVs as compared to healthy subjects [46]. These EVs are also associated with atherosclerosis by inducing hypercoagulation, inflammation and cell adhesion [47,48].
Leukocyte-derived EVs can originate from neutrophils, monocytes/macrophages, and lymphocytes, as differentiated by specific markers associated with their parental cells [49]. EVs released by leukocytes may have an important role in maintaining or disrupting vascular homeostasis and pathological thrombosis contributing to inflammatory responses [49]. T cell-derived EVs were increased in the circulation of an animal model of angiotensin II (ANG II)-induced hypertension, resulting in inflammatory response [50]. Plasma levels of the leukocyte-derived EVs were elevated in patients with hypertension and hyperlipidemia [51]. Melnikov et al. [52] identified monocytes-derived EVs carrying monomeric C-reactive protein (mCRP) in the blood that was associated with inflammatory status in CAD patients.
2.2. Heart Cell- and Blood Vessel-Derived EVs
It has been reported that EVs could be released from major cell types in the heart [15,53], such as cardiomyocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells (SMC). Loyer et al. [54] demonstrated, using a murine model of myocardial infarction, that EVs released by cardiomyocytes and endothelial cells following myocardial infarction could be taken up by monocytes and regulate the cardiac inflammatory response by releasing of proinflammatory cytokines.
Endothelial EVs are associated with the progression of atherosclerosis [55], hypertension [51], and CAD [56]. On the other hand, EVs can play protective roles. For example, endothelial cell-derived EVs were reported as cardioprotective molecules releasing proteins involved in cellular homeostasis and preservation in the ischemia-reperfusion injury in a chip model of human heart [57]. Several studies were summarized in a review that reported activated endothelial cell-derived EVs were also involved in the regulation of cardiac and vascular remodeling in HF [58].
Cardiomyocyte-derived EVs take an important part in the progression of CVD, because they can carry a wide variety of biomolecules, such as proteins and miRNAs, to other cell types and regulate the function and gene expression in these cells [59], especially promoting cardiac repair [60]. Cardiomyocyte-derived EVs secreted from primary cardiomyocytes and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) can have angiogenic effects after myocardial infarction through inducing increase expression of miRNAs and proteins, such as growth factors [61,62], also inducing cardiac fibrosis by release of specific miRNAs via myocyte-fibroblast cross-talk [63]. EVs released from cardiomyocytes derived from human-induced pluripotent stem cells were also used in treatment of heart injury, including myocardial infarction, contributing to cardiac regeneration, through cardiac-specific miRNAs activity [64,65].
Cardiac fibroblast-derived EVs stimulated cell cardiac migration [66], SMC proliferation, and vascular remodeling [67] by release of miRNAs. Vascular SMC-derived EVs are enriched with RNAs, proteins and lipids associated with vascular remodeling, calcification and coagulation [68,69,70,71,72], familial hypercholesterolemia, and CAD [73]. Most studies have evaluated changes in SMC caused by EVs derived from other cells. For example, EVs derived from bone marrow mesenchymal stem cells (BMSC) have been shown to induce calcification in vascular SMC by modifying miRNA profiles [74], and EVs derived from platelets could modulate inflammatory response in vascular SMC by presenting chemokine CXCL4 and membrane-bound effectors [75].
2.3. EVs Interaction between Cells from Different Origins
The role of EVs in intercellular communication and interaction between heart-derived cells was reviewed by Hafiane et al. [8], and EV communication between platelets, monocytes, and endothelial cells was associated with myocardial ischemia. Weiss et al. [76] reported differential interaction of platelet-derived EVs with monocytes and other leukocytes, which were identified by specific markers using flow cytometry. The authors used CD41 as marker of platelet origin, CD45-PB and CD14-PE as monocyte markers, CD16/56-PC5 as granulocyte and NK cell marker, and CD3-ECD as T cell marker.
Quiescent endothelial cells were shown to release EVs that were able to suppress monocyte activation and anti-inflammatory molecules associated with vascular inflammation in CVD [77]. TNF-α-induced inflamed endothelial cells were shown to release EVs enriched in cytokines, chemokines and other inflammatory markers, which when transferred to monocytes promoted their differentiation to pro- or anti-inflammatory phenotypes [78].
EVs derived from macrophage foam cells from patients with atherosclerosis were shown to integrate into vascular SMC and induce their migration and adhesion [79]. EVs also participate in communication between endothelial and vascular SMC. Boyer et al. [80] demonstrated that endothelial-derived EVs could also stimulate protein synthesis and senescence of vascular SMC. In addition, a recent study reported EV-mediated transmission of RNA between endothelial cells and SMC, alleviating ANG II-induced vascular dysfunction [81].
3. Extracellular Vesicles Quantification as Biomarker in CVD
Several studies have shown an association of circulating EV counts with CVD, suggesting a potential application of EV quantification as a biomarker for diagnostic and therapeutic monitoring [25,82,83]. Although using EV counts from particular cell type as biomarker seems promising, the major limitation of this approach is the lack of standardization of methods, resulting in difficulty to compare studies from multiple research groups [25].
The release of platelet-derived EVs was shown to be increased in plasma, under conditions with enhanced platelet activation, such as myocardial infarction and exposure to modified lipoproteins [33,84]. Likewise, in arterial and venous thrombosis, the activated platelets increase the circulating EV counts compared with healthy condition [25].
Patients with atherothrombotic diseases and atherosclerotic lesions have high levels of circulating EVs derived from endothelial cells, vascular SMC, platelets, leukocytes or erythrocytes [85]. Sansone et al. observed an increase of endothelial-derived EVs in the plasma of patients with arterial hypertension with and without CAD [56]. Plasma levels of leukocyte-derived EVs were reported to be increased in atherosclerotic patients, and they were correlated with the progression of the atherosclerosis [79].
Several studies have reported increased counts of EVs in ACS conditions [86,87,88,89,90]. Serum EVs were found to be higher in patients with STEMI than whose with stable angina or control subjects, suggesting early stages increases in the disease due to thrombus formation and ischemia-induced stress [91]. Erythrocyte-derived EV counts were also elevated in STEMI patients [46].
Importantly, the increase of circulating EVs can be detected shortly after the pathological stimulus. Deddens et al. [92] demonstrated that plasma EVs are rapidly detectable. In one study, the amount of EVs was already increased one hour after myocardial infarction. Ge et al. [93] observed a significant increase in heart tissue EVs release 24 h after myocardial ischemia/reperfusion (I/R).
Patients with persistent atrial fibrillation (AF) and a high level of inflammation showed markedly increased EV concentration compared to subjects without AF [82]. In addition, the inflammation contributes to platelet activation that induces the release of EVs in a prothrombotic state [82]. A recent study also showed that circulating EVs were increased in patients with AF and a higher risk of stroke than non-AF patients of similar age [94].
Circulating EVs derived from endothelial cells were explored in a prospective study, which demonstrated that patients with HF had increased plasma levels of endothelium-derived microparticles compared to healthy subjects [95]. These HF patients had a higher probability of cardiovascular events (e.g., cardiovascular death, non-fatal myocardial infarction, ischemic stroke, or re-hospitalization related to HF), and it was suggested that EV counts could be a useful prognostic biomarker. Patients with symptoms of chronic HF had increased number of circulating endothelial-derived EVs that were correlated with increase of mortality and recurrent hospitalization risk due to HF [96]. HF patients also had increased serum levels of EVs compared to healthy subjects [97]. A recent review [58] has reported that the number of EVs might be important to differentiate the severity of HF.
Circulating EV counts are also altered in patients with metabolic disorders that increase the risk of CVD. For example, the total number of circulating EVs was shown to be higher in patients with metabolic syndrome (MetS) compared to non-MetS subjects [98]. Increased levels of endothelial-derived EVs were also observed in diabetic patients compared with healthy controls, and they were closely associated with vascular dysfunction [99]. Circulating levels of lymphocyte-derived EVs were also increased in patients with familial hypercholesterolemia [100].
4. Extracellular Vesicle as Biomarkers in CVD
Studies on the important regulatory effects of EVs in CVD has been motivated due to EV stability, their specific signatures associated with cell activation or injury, and their intrinsic activity and immunomodulatory properties [13]. The changes in EV cargo, including RNAs, proteins, and lipids, as potential biomarkers in CVD are reviewed in Table 1.
Table 1.
Summary of extracellular vesicles (EV) cargo as biomarkers in cardiovascular disease.
| EV Cargo | Source | Disease | EV Isolation | EV Characterization | Quantification Methods | Clinical Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| RNAs | |||||||
| lncRNA Neat1 | Cardiomyocytes | Cardiac ischemia | Ultracentrifugation | Western blot; NTA | qRT-PCR | lncRNA Neat1 EV modulates the expression of P53 target genes, cell-cycle regulators and promoted cellular survival. | [101] |
| miR-126 miR-199a |
Plasma | CAD | Ultracentrifugation | Flow Cytometry | qRT-PCR | Increased plasma EV miR-126 and miR-199a reduce the risk of major cardiovascular outcomes in CAD patients | [102] |
| miR-126 | Plasma | High-risk CVD | Ultracentrifugation / magnetic beads | TEM; NTA | qRT-PCR | EV miR-126 plasma levels are negatively correlated with NT-proBNP and cTnI. miR-126 as a potential biomarker of CVD | [103] |
| miR-30 emiR-92a |
Plasma | Coronary atherosclerosis | ExoQuick Exosome Precipitation kit (SBI) | − | qRT-PCR | High plasma EV miR-30e and miR-92a, which regulate ABCA1, as new biomarkers for clinical diagnosis and treatment of coronary atherosclerosis | [104] |
| miR-208a | Serum | ACS | ExoQuick Exosome Precipitation kit (SBI) | Western blot | qRT-PCR | Increased serum EV miR-208 is related to early diagnosis and prognosis of ACS | [105] |
| miR-34a miR-192 miR-194 |
Serum | HF | ExoQuick Exosome Precipitation kit (SBI) | Western blot | qRT-PCR | Increased serum EV miR-34a, miR-192 and miR-194 are predictive of HF after AMI | [106] |
| miR-92b-5p | Serum | HF | Exosome isolation kit (RiboBio) | NTA; TEM; Western blot | qRT-PCR | Increased serum EV miR-92b-5p as biomarker for diagnosis of acute HF | [107] |
| miR-155 | Urine | CAD | Ultracentifugation | NTA; TEM; Flow cytometry | qRT-PCR | Increased urinary EV miR-155 as a biomarker of CAD progression | [108] |
| miR-92a | Endothelial cells | CAD | Ultracentrifugation | Flow cytometry | qRT-PCR | EC-derived EV miR-92a is increased in CAD patients. miR-92a regulates angiogenesis in recipient EC | [109] |
| miR-92a | Endothelial cells | Atherosclerosis | Ultracentrifugation | TEM; NTA; Western blot | qRT-PCR | EC-derived EV miR-92a as potential therapeutic target in atherosclerosis-related diseases | [110] |
| miR-128 miR-146 amiR-185 miR-365 miR-503 |
Macrophages | Atherosclerosis | ExoQuick-TC Exosome Precipitation kit (SBI); Ultracentrifugation | NTA; Western blot | Affymetrix miRNA 3.0 microarray; qRT-PCR | EV-derived miRNAs secreted by atherogenic macrophages may accelerate atherosclerosis | [111] |
| Proteins | |||||||
| CD31/Annexin 5 | Plasma | CAD | PE-conjugated anti-CD31 and FITC-conjugated anti-annexin 5 | Flow cytometry | Flow cytometry | Increased plasma CD31/Annexin 5 EVs as an independent predictor of cardiovascular events in CAD patients | [112] |
| C1Q1A C5 GP1BA PPBP APOD APOC3 |
Plasma | Myocardial infarction | Ultracentrifugation | Western blot; Cryo-EM | LC-MS/MS | Plasma EV proteins as predictive biomarkers and therapeutic targets in myocardial infarction | [113] |
| CD144 | Plasma | Myocardial injury | Ultracentrifugation | Flow cytometry | Flow cytometry | Increased plasma of CD144-EVs as predictor of cardiovascular complications | [114] |
| SerpinC1SerpinG1CD14 Cystatin C | Plasma | IHD | Ultracentrifugation | Western blot; TEM; NTA | Bio-plex 200 systems (Bio-Rad) | Plasma EV proteins are associated with stable IHD | [115] |
| Cystatin C CD14 SerpinG1 SerpinF2 |
Plasma | HF | OptiPrep™ Density Gradient Medium; Ultracentrifugation | Western blot; TEM | Quantitative Magnetic Bead Assays | Plasma levels of EV CD14, SerpinG1 and SerpinF2 are associated with HF | [116] |
| Cystatin C pIgR C5a |
Serum | ACS | ExoQuick exosome precipitation kit (SBI) | − | Luminex- based multiplex panels | Serum concentrations of EV protein are associated with ACS | [117] |
| mCRP | Monocytes | CAD | Exo-FLOWTM exosome capture kit | Flow cytometry | Flow cytometry | mCRP in monocyte-derived EVs as biomarker of inflammatory process in CAD patients | [52] |
| mCRP | Endothelial cells | Myocardial infarction | Ultracentrifugation | Flow cytometry | Western blot; Flow cytometry | EV transport and delivery of pro-inflammatory mCRP in AMI patients | [118] |
| mCRP | Endothelial cell | PAD | Ultracentrifugation | Flow cytometry; TEM | ELISA; Western blot | EC-derived EV mCRP is increased in patients with PAD, and was suggested as a predictor of vascular disease risk | [119] |
| ANXA1 | Valvular interstitial cells | − | Ultracentrifugation | NTA; TEM; ExoView R100 platform | LC-MS/MS | ANXA1 induces EV aggregation and microcalcification formation and was suggested as a therapeutic target | [120] |
| CD11b CD16 CD45 |
Urine | CAD | Ultracentifugation | NTA; TEM; Flow cytometry | Flow cytometry | Increased CD45+ and CD11b+ and decreased CD16+ in urinary EVs are associated with CAD progression | [108] |
| Nephrin Podocalyxin |
Urine | Hypertension | Total Exosome Isolation kit (Invitrogen) | Flow cytometry | Flow cytometry | Urinary levels of EVs enriched in nephrin and podocalyxin are increased in hypertensive patients |
[121] |
| p16 | Urine | Hypertension | Total Exosome Isolation kit (Invitrogen) | Flow cytometry | Flow cytometry | Urinary p16 EVs are increased in hypertensive patients | [122] |
| Lipids | |||||||
| Sphingolipid (ceramides, dihydroceramides, and sphingomyelins) | Plasma | STEMI | Ultracentrifugation | NTA, Flow cytometry; Western blot | LC-MS/MS | EV lipid signature discriminates STEMI patients and may be used as therapeutic strategy | [123] |
| Phosphatidylserine | Platelet | − | Centrifugation | Flow cytometry; Western blot, TEM | Flow cytometry | EV phosphatidylserine may contribute in thrombin generation and promoting thrombosis | [38] |
| Metabolites | |||||||
| 4-aminohippuric acid Citric acid N-1-methylnicotinamide |
Urine | CVD | Ultracentrifugation | TEM; Western blot | SRM-LC-MS/MS | Urinary EV metabolite deregulation as biomarker of CVD | [124] |
ABCA1: ATP binding cassette (ABC)A1; ACS: acute coronary syndrome; AMI: acute myocardial infarction; ANXA1: Annexin A1; C5a: complement factor C5a; CAD: coronary artery disease; Cryo-EM: Cryo-electron Microscopy; cTnI: cardiac troponin I; CVD: cardiovascular disease; EC: endothelial cells; FITC: fluorescein isothiocyanate; HF: heart failure; IHD: Ischemic heart disease; LC-MS/MS: liquid chromatography coupled to tandem mass spectrometry; mCRP: pro-inflammatory monomers; NTA: nanoparticle tracking analysis; NT-proBNP: N-terminal propeptide of B-type natriuretic peptide; PAD: peripheral artery disease; PE: Phycoerythrin; pIgR: polygenic immunoglobulin receptor; qRT-PCR: reverse transcription quantitative polymerase chain reaction; SBI: System Biosciences; SRM-LC-MS/MS: Target mass spectrometry in selected reaction monitoring mode, coupled to liquid chromatography; STEMI: ST-segment-elevation myocardial infarction; TEM: transmission electron micrographs.
4.1. Extracellular Vesicles Carrying RNAs
The EV transcriptome of various cell types is important due to the biological relevance of RNA activity in several cardiovascular pathologies [58,109,125,126]. EVs are carriers of various RNA types, such as messenger RNA (mRNA), transfer RNA (tRNA), small interference RNA (siRNA), long-non-coding RNA (lncRNA), and microRNA (miRNA) [13]. An earlier study identified mRNAs and miRNAs in EVs by microarray technology and showed the transference of functional RNA between three cell lines [127].
Kenneweg et al. [101] showed lncRNA-enriched EVs in cardiac ischemia. In this context, lncRNA Neat1 was necessary for fibroblast and cardiomyocyte survival, and the silencing of Neat1 resulted in reduced heart function after myocardial infarction. A study identified 185 differentially expressed circular RNAs (circRNAs), covalently closed RNAs, involved in the metabolic process from EVs of the murine heart post-I/R injury compared with control, and these circRNAs may regulate target genes by acting on the miRNAs [93].
miRNAs are short non-coding RNAs (19-22 nucleotides) that regulate gene expression at the post-transcriptional level by binding to specific mRNAs with varying degrees of complementarity and leading to mRNA degradation and/or translational inhibition [128,129]. miRNAs control different physiological processes and abnormal patterns of expression have already been associated with many diseases [129]. Different types of cells can release miRNAs into the extracellular space in response to various stimuli and pathologies [130,131]. In peripheral circulation, EVs are responsible for protecting miRNAs from degradation by circulating ribonucleases [130,132].
The EV-miRNAs can be promising predictors or indicators for premature CVD detection. Increased expression of miR-126 and miR-199a isolated from circulating EVs was proposed to reduce the risk of major cardiovascular outcomes in patients with CAD [102]. Cheng et al. [133] suggested that expression of miR-126 and miR-21 could be used for early detection of CVD, such as acute myocardial infarction and FH. Another study reported reduced plasma levels of EV-miR-126 in high-risk CVD patients and EV-miR-126 levels were negatively correlated with cardiac troponin I (cTnI) and N-terminal propeptide of B-type natriuretic peptide (NT-proBNP), suggesting miR-126 as a potential biomarker for CVD [103].
miRNA-208a expression was upregulated in the serum exosomes of ACS patients, and the study suggested its important role for the early diagnosis and prognosis of ACS [105]. Two EV-miRNA (miR-30e and miR-92a) that target ATP binding cassette (ABC)A1 were shown to be upregulated in plasma EVs from patients with coronary atherosclerosis [104]. Endothelial cells-derived EVs containing miR-92a were increased in patients with CAD, and this miRNA was shown to regulate angiogenesis in recipient endothelial cells [109]. EV-enriched miR-92a can be transferred from endothelial cells to macrophages and suppress Kruppel-like factor 4 (KLF4) expression in recipient cells, resulting in an atherosclerotic phenotype [110,134]. In addition, upregulation of the miR-1 in hepatocyte-derived EVs was associated with promotion of endothelial inflammation and facilitate atherogenesis by downregulation of KLF4 and activation of the NF-κB [135].
Increased levels of urinary EVs miRNAs were reported in patients with unstable CAD compared to whose with stable CAD [108]. The authors suggested an important role of miR-155 in disease progression that could be used as prognostic indicator and therapeutic target.
Atherogenic EVs from mouse and human macrophages were enriched in miR-146a, miR-128, miR-185, miR-365, and miR-503. Further, miR-146a was related to progression of atherosclerosis by decreasing cell migration and promoting macrophage entrapment in the vessel wall [111].
Elevated expression of miR-192, miR-194 and miR-34a in serum EVs was observed in HF patients after acute myocardial infarction [106]. Serum exosomal miR-92b-5p was increased in patients with HF due to dilated cardiomyopathy ant this miRNA was suggested as biomarker for diagnosis of HF [107].
EV miRNAs were also related to cardiovascular risk factors (i.e., diabetes, dyslipidemia, obesity, MetS). Cardiomyocytes isolated from type 2 diabetic rats had inhibitory effects on myocardial angiogenesis through the EV transfer of miR-320 into endothelial cells [136]. miR-24 and miR-130a were downregulated in plasma EVs of patients with familial hypercholesterolemia (FH), and miR-130a levels were inversely related to coronary atherosclerosis in suspected CAD patients, suggesting their role as potential biomarkers of FH and CAD [73].
A recent study using abdominal adipose tissue-derived mesenchymal stem/stromal cells showed four downregulated miRNAs (miR-136, miR-4798, miR-12,136, miR-222) and nine upregulated miRNAs (miR-630, miR-144, miR-143, miR-4787, miR-769, miR-8074, and miR-181a) from EVs of MetS patients. These deregulated miRNAs might control genes, which were associated with cellular senescence, cell cycle, metabolic processes, and apoptosis pathways [137].
4.2. Extracellular Vesicles Carrying Proteins
Differences in EV protein levels occur in response to a variety of physiological or pathological stimuli. The protein profile might change already in a very early stage of the disease, which makes this content a potential early biomarker [115]. The EV protein cargo is heterogenous and dependent on the cell type or biofluid of origin [138].
EV proteins were suggested to be prognostic biomarkers of cardiovascular events. In this context, a prospective study demonstrated the increase of circulating CD31/Annexin 5-positive EVs as an independent predictor of cardiovascular risk in patients with stable CAD. High levels of CD31/Annexin 5 EVs were associated with higher incidence of death caused by CVD and higher need for revascularization [112,139].
A study of EV proteome of patients with myocardial infarction identified six novel EV protein markers of myocardial damage related to three pathways: complement activation (C1Q1A and C5), platelet activation (GP1BA and PPBP), and lipid metabolism (APOD and APOC3) [113]. Increased plasma levels of CD144-EVs were also suggested to be predictive of cardiovascular complications (i.e., ACS, ischemic stroke, revascularization, and death) in patients with high risk for CAD [114,139].
The link between EV proteins and atherosclerosis was described in a study, which showed that hypercholesterolemic patients with subclinical lipid-rich atherosclerotic plaques have a higher abundance of CD45/CD3-derived EVs than those in patients with fibrous plaques [100].
EV protein levels showed an association with stress-induced ischemia, especially proteins known to be related to inflammatory cascades such as SerpinC1, SerpinG1, CD14, and Cystatin C [115]. Serum EV proteins, such as cystatin C, polygenic immunoglobulin receptor (pIgR) and complement factor C5a (C5a), were suggested to be associated with ACS [117,140]. mCRP carried by monocyte-derived EVs was associated with inflammatory process in patients with CAD [52]. EVs can transport and delivery pro-inflammatory mCRP in endothelial cells [118]. mCRP carried by endothelial cell-derived EVs was also increased in patients with peripheral artery disease, and it was suggested to be a pro-inflammatory molecule and a potential indicator of vascular disease risk [119].
Plasma levels of EVs enriched in cystatin C, CD14, serpinG1, and serpinF2 were markedly increased in HF patients. These EVs proteins, previously related to systemic vascular events, were associated with high risk of HF in patients suspected of acute HF [116].
A recent study, using human cardiovascular cells, demonstrated that Annexin A1 induces EVs aggregation and microcalcification formation that promote CVD. These findings could lead to development of therapeutic strategies in CVD [120].
Urinary levels of EV proteins were decreased in patients with unstable CAD, however levels of CD45+ and CD11b+ EVs were increased and CD16+ EVs were decreased. These urinary EV proteins were suggested to be associated with CAD progression [108]. High levels of urinary EVs enriched in nephrin and podocalyxin were observed in patients with hypertension and these EV proteins were proposed to be useful diagnostic biomarkers [121]. Urinary EVs released by senescent nephron cells had increased levels of p16 (senescence marker) in patients with hypertension as compared to healthy volunteers. Urinary p16-positive EVs could serve as an early marker of nephron senescence and could be useful in disease management and therapeutic follow-up [122].
4.3. Extracellular Vesicles Carrying Lipids and Metabolites
Lipids are important components of vesicle bilayer membranes and specifics lipids, such as cholesterol and sphingomyelin, are enriched in vesicles compared to their parental cells and it might modulate recipient cell homeostasis [18,141]. Then, lipids are emerging as very important players for the physiological functions of these vesicles [142]. The first studies relating to the lipid composition of EVs were performed on prostate-derived EVs found in seminal fluid about twenty years ago [142,143]. The data have been included in the EVs databases such as Vesiclepedia [144], EVpedia [145], and Exocarta [146].
EVs lipids interact with receptors on the target cell and are thereafter internalized intro endosomes where they concentrate the bioactive lipids that they carry modulating endogenous cell lipid metabolism [18]. Since lipids are essential structural and functional constituents of EVs [142], the use of EV lipids as biomarkers of CVD may be promising, however, there are only a few studies on this topic.
EVs can carry ceramides, sphingomyelin, lysophosphatidylcholine, arachidonic acid, and other fatty acids, cholesterol, prostaglandins, leukotrienes, and active lipolytic enzymes (such as phospholipase A2) on their membrane or within their lumen, and their lipid composition can be modified by in vitro manipulation [18]. Circulating EVs were enriched with different sphingolipid species (ceramides, dihydroceramides, and sphingomyelins) in patients with STEMI, and lipid levels correlated with cardiac troponin, leucocyte count, and lower left ventricular ejection fraction [123].
The amount of lipids in the shed EVs could be directly related to atherosclerosis, once accumulation of these lipids was associated with foam cell formation and apoptosis in macrophages mediated by toll-like receptors, which can lead to atherosclerosis [8,147]. EVs can be released by activated platelets, which are rich in phosphatidylserine, contributing in thrombin generation and promoting thrombosis [38,148]. Activated platelets also release EVs rich in arachidonic acid, which contributes to thrombosis in the recipient cells by the promotion of the cell adhesion and stimulation of prostacyclin and thromboxane A2 synthesis [149,150].
A pioneering study showed that urinary EV metabolites (4-aminohippuric acid, citric acid, and N-1-methylnicotinamide) were altered in patients with high cardiovascular risk. Urinary EV levels of 4-aminohippuric acid were increased, whereas citric acid and N-1-methylnicotinamide were reduced in patients with high cardiovascular risk, suggesting an important role of EV metabolites as biomarkers of CVD [124].
5. Extracellular Vesicles as Biomarkers for Therapeutic Responses in CVD
Plasma EV counts have been explored as biomarkers to assess the response to cholesterol-lowering and antiplatelet therapies. Suades et al. [151] showed a reduction in the number of circulating EVs, specifically microparticles, derived from endothelium, platelets, and inflammatory cells after lipid-lowering therapy with statins. Kulshreshtha et al. [83] also described that simvastatin reduced the secretion of EVs from various cell types. Conversely, atorvastatin was shown to increase the number of circulating endothelial-derived EVs in patients with peripheral arterial occlusive disease [152]. In the same way, Zu el al. [51] showed that lipid-lowering and antihypertensive therapies increased plasma levels of endothelial-derived EVs. Consequently, these EVs reduced the adhesion molecules of monocytes to endothelial cells, such as VCAM-1 and ICAM-1, resulting in improvement of the endothelial function.
Platelet P2Y12 receptor inhibitors or antagonists, such as clopidogrel and ticagrelor, were suggested to alter the EV counts in plasma. Platelet- and leukocyte-derived EV levels were reported to be lower in patients taking ticagrelor compared to clopidogrel after acute myocardial infarction [153]. The authors suggested that reduction of EVs may explain better clinical outcomes with less thrombotic events in ticagrelor compared to clopidogrel. Chyrchel et al. [154] showed that prasugrel and ticagrelor have higher antiplatelet effect compared with clopidogrel because they decrease plasma levels of platelet-derived EVs. The nitrate supplementation reduced platelet-derived EVs, increasing the response to clopidogrel in CAD patients and it may represent a novel therapeutic strategy to reduce the risk of thrombosis in these patients [155].
6. Extracellular Vesicles as Drug Delivery Vehicles in CVD
EVs can incorporate bioactive molecules, act in intercellular communication and have a therapeutic potential, these characteristics have been explored for the use of these vesicles as drug delivery system [13]. EVs may offer high delivery efficiency, intrinsic targeting properties, and low mutagenicity [156]. In addition, the use of EVs as drug delivery vehicle is beneficial as it associates with low immunogenicity, because EVs are biologically produced and have low toxic effects compared with foreign molecules, such as virus-derived vehicles, or cell therapies [157]. Together, these aspects consider the EVs as safe delivery tool.
For the development of the drug delivery system, the bioactive molecule can be loaded into vesicles during production phase by co-incubation in the cell culture or can be incorporated after the production and isolation of the EVs. Nucleic acids and proteins can be loaded by transfecting the producing cell with the encoding DNA inserted into a vector [158].
Based on the ability of EVs to transfer their contents to cells and tissues, circulating EVs involved in cardiovascular protection have been studied, mainly for the delivery of therapeutic miRNAs [158,159]. An in vitro study showed that EV-derived cardiac endothelial cells from ischemic myocardium overexpressing hypoxia-inducible factor-1 had higher content of miR-126 and miR-210. These EVs transferred the miRNAs to cardiac progenitor cells and increased the tolerance to hypoxic stress, a protective effect of EVs [160].
In apolipoprotein E (apoE)-deficient mice, inhibition of EV-mediated miR-155 transfer from SMC to endothelial cells, using anti-miR-155, reduced the endothelial injury and atherosclerosis, suggesting a promising therapy for atherosclerotic patients [161]. In an animal model (C57BL/6 mice) of myocardial infarction, miRNA-21-loaded EVs were internalized in cardiomyocytes and endothelial cells, restoring the cardiac function [162]. Mesenchymal stem cell-derived EVs were shown to inhibit atherosclerotic plaque formation by delivery of miR-221 to vascular SMC [163].
Proteins-derived EVs also have been reported in cardiovascular protection. Vicencio et al [164] demonstrated that EVs loaded with HSP70 had cardioprotective effects in ex vivo, in vivo, and in vitro settings of cardiac ischemia-reperfusion injury. The mechanism involves the stimulation of the toll-like receptor (TLR) 4 by HSP70 and various kinases leading to HSP27 phosphorylation in cardiomyocytes. Leukocyte/platelet-derived EVs were reported to mediate anti-inflammatory effects by downregulation of pro-inflammatory genes [165].
A recent study evaluated the anti-atherosclerotic effect of platelet-derived EVs loaded with MCC950, an NLRP3-inflammasome inhibitor. MCC950-loaded EVs were administrated intravenously and reduced the inflammatory process, the formation of atherosclerotic plaque and inhibited the proliferation of macrophages and T cells in apoE-deficient mice [166]. Decrease of the inflammatory process in atherosclerosis was reported in a study that used molecularly engineered ani-inflammatory M2 macrophage-derived exosomes, and further electroporated with FDA-approved hexyl 5-aminolevulinate hydrochloride (HAL). This study suggested the use of the HAL-engineered M2 macrophage-derived exosomes for atherosclerosis and inflammation-associated diseases therapy [167].
The yield of EV isolation is a methodological limitation. A study reported that pH acid (pH4) could be an effective environment to isolate EVs because it increases the levels of EV content, such as RNA and protein (including EV markers), while in alkaline condition (pH11) no EV RNA and proteins have been detected [168]. The yield of EVs can be also affected by storage temperature [169] and solvent (storage buffers, such as Phosphate-buffered saline, Sodium chloride) [157]. The generation of EV mimetics (EVMs) could serve as an important strategy to improve the use of EVs as a novel drug delivery system, using for example to delivery siRNA (siRNA-loaded EVMs) with better yield [156,170,171]. EVMs are vesicles produced artificially from cells or by mixing various lipid compositions similar to EVs. This can be a promising drug delivery vehicle because it maintains the intercellular communication by releasing nucleic acids, proteins, and lipids between cells [172], and can be an important strategy for scale-up production EVs in a short period of time [173].
A previous study suggested that EV uptake in the cells occurs by clathrin-independent endocytosis and micropinocytosis [174]. An important point in the development of efficient EV-based drug delivery therapy is the identification of components on the EV surface that allows their internalization and consequent transfer of their internal cargo to the recipient cells.
To the best of our knowledge, EV delivery approach has not been approved for the treatment of CVD yet. There are more than 50 nanomedicines approved by FDA for some diseases, mainly cancer [175]. These systems use liposomes, polymeric nanoparticles, and inorganic nanoparticles, which have similar to exosome size [176]. EVs as drug delivery system is a technology that offers the opportunity for the development of new pharmacological therapies, but it still needs to be further explored to solve the yield and delivery-associated issues.
7. Conclusions
The great potential of using EVs as a tool to study the pathophysiology of variety of CVD was addressed in this review. The relevance of EVs in intercellular communication and aspects of cellular origin, quantification, and composition of circulating EVs were also explored. Circulating EVs were discussed as potential biomarkers for the diagnosis, prognosis and therapeutic monitoring in CVD, and their risk factors such as metabolic diseases. EVs as biomarkers in CVD seem not so far away to be used in clinic setup. This field is evolving rapidly, and scientists are constantly improving the techniques for isolation, characterization, and analysis of EVs. EVs also have a promising application as a drug delivery system for CVD therapies once technical limitations could be overcome. Future studies on EV composition using more sensitive tools would increase the spectrum of EV clinical applications.
Author Contributions
R.C.C.d.F. drafted the manuscript; R.D.C.H. and M.H.H. critically revised and edited the manuscript. E.A. conceptualized, and critically revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
R.C.C.d.F. is a recipient of a fellowship (grant#2019/22147-0) from Sao Paulo Research Foundation (FAPESP). R.D.C.H. and M.H.H. are recipients of fellowships from National Council for Scientific and Technological Development (CNPq), Brazil. E.A. research is supported by National Institutes of Health grants R01 HL136431, R01 HL147095, and R01 HL141917.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 3.Cocucci E., Meldolesi J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 5.La Salvia S., Gunasekaran P.M., Byrd J.B., Erdbrügger U. Extracellular Vesicles in Essential Hypertension: Hidden Messengers. Curr. Hypertens. Rep. 2020;22 doi: 10.1007/s11906-020-01084-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 7.Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., Zimmerman A., Amiji M., Ivanov A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020;38:1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafiane A., Daskalopoulou S.S. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism. 2018;85:213–222. doi: 10.1016/j.metabol.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra H., Drummen G.P.C., Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royo F., Théry C., Falcón-Pérez J.M., Nieuwland R., Witwer K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells. 2020;9:1955. doi: 10.3390/cells9091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari S., Kumar V., Randhawa S., Verma S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2020:e13367. doi: 10.1111/aji.13367. [DOI] [PubMed] [Google Scholar]
- 13.Chong S.Y., Lee C.K., Huang C., Ou Y.H., Charles C.J., Richards A.M., Neupane Y.R., Pavon M.V., Zharkova O., Pastorin G., et al. Extracellular vesicles in cardiovascular diseases: Alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int. J. Mol. Sci. 2019;20:3272. doi: 10.3390/ijms20133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen F., Li Q. Advances in Experimental Medicine and Biology. Vol. 998. Springer New York LLC.; New York, NY, USA: 2017. Exosomes as diagnostic biomarkers in cardiovascular diseases; pp. 61–70. [DOI] [PubMed] [Google Scholar]
- 15.Sluijter J.P.G., Davidson S.M., Boulanger C.M., Buzás E.I., De Kleijn D.P.V., Engel F.B., Giricz Z., Hausenloy D.J., Kishore R., Lecour S., et al. Extracellular Vesicles in Diagnostics and Therapy of the Ischaemic Heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Vol. 114. Oxford University Press; Oxford, UK: 2018. pp. 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bei Y., Das S., Rodosthenous R.S., Holvoet P., Vanhaverbeke M., Monteiro M.C., Monteiro V.V.S., Radosinska J., Bartekova M., Jansen F., et al. Extracellular vesicles in cardiovascular theranostics. Theranostics. 2017;7:4168–4182. doi: 10.7150/thno.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.New S.E.P., Aikawa E. Role of Extracellular Vesicles in De Novo Mineralization. Arterioscler. Thromb. Vasc. Biol. 2013;33:1753–1758. doi: 10.1161/ATVBAHA.112.300128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Li G., Liu M.L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics, Proteomics Bioinforma. 2018;16:50–62. doi: 10.1016/j.gpb.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen F., Nickenig G., Werner N. Extracellular vesicles in cardiovascular disease. Circ. Res. 2017;120:1649–1657. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 21.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaptoge S., Pennells L., De Bacquer D., Cooney M.T., Kavousi M., Stevens G., Riley L.M., Savin S., Khan T., Altay S., et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health. 2019;7:e1332–e1345. doi: 10.1016/S2214-109X(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 24.Fu S., Zhang Y., Li Y., Luo L., Zhao Y., Yao Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020;6 doi: 10.1038/s41420-020-00305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickhout A., Koenen R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018;5:113. doi: 10.3389/fcvm.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripisciano C., Weiss R., Karuthedom George S., Fischer M.B., Weber V. Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020;8:298. doi: 10.3389/fcell.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caroline R., De Freitas C., Bortolin R.H., Vecchia F.D., Medina-pestana O., Cerda A., Doi S.Q. Differentially expressed urinary exo-miRs and clinical outcomes in kidney recipients on short-term tacrolimus therapy: A pilot study. Epigenomics. 2020;12:2019–2034. doi: 10.2217/epi-2020-0160. [DOI] [PubMed] [Google Scholar]
- 28.Iwai K., Minamisawa T., Suga K., Yajima Y., Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J. Extracell. Vesicles. 2016;5:30829. doi: 10.3402/jev.v5.30829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza A.H., Kaur S., Nielsen L.B., Størling J., Yarani R., Roursgaard M., Mathiesen E.R., Damm P., Svare J., Mortensen H.B., et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes From Mothers With Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019;10:2543. doi: 10.3389/fimmu.2019.02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vojtech L., Zhang M., Davé V., Levy C., Hughes S.M., Wang R., Calienes F., Prlic M., Nance E., Hladik F. Extracellular vesicles in human semen modulate antigen-presenting cell function and decrease downstream antiviral T cell responses. PLoS ONE. 2019;14:e0223901. doi: 10.1371/journal.pone.0223901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Freitas R.C.C., Bortolin R.H., Lopes M.B., Tamborlin L., Meneguello L., Silbiger V.N., Hirata R.D.C., Hirata M.H., Luchessi A.D., Luchessi A.D. Modulation of miR-26a-5p and miR-15b-5p Exosomal Expression Associated with Clopidogrel-Induced Hepatotoxicity in HepG2 Cells. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh S., Mizuno K., Aikawa M., Aikawa E. Dimerization of sortilin regulates its trafficking to extracellular vesicles. J. Biol. Chem. 2018;293:4532–4544. doi: 10.1074/jbc.RA117.000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puhm F., Boilard E., Machlus K.R. Platelet Extracellular Vesicles. Arterioscler. Thromb. Vasc. Biol. 2020 doi: 10.1161/ATVBAHA.120.314644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenen R.R., Aikawa E. Editorial: Extracellular Vesicle-Mediated Processes in Cardiovascular Diseases. Front. Cardiovasc. Med. 2018;5:133. doi: 10.3389/fcvm.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaldivia M.T.K., McFadyen J.D., Lim B., Wang X., Peter K. Platelet-Derived Microvesicles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2017;4:74. doi: 10.3389/fcvm.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z.T., Wang Z., Hu Y.W. Possible roles of platelet-derived microparticles in atherosclerosis. Atherosclerosis. 2016;248:10–16. doi: 10.1016/j.atherosclerosis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Sun C., Zhao W.B., Chen Y., Hu H.Y. Higher Plasma Concentrations of Platelet Microparticles in Patients With Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2016;32:1325.e1. doi: 10.1016/j.cjca.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 38.Wei H., Malcor J.D.M., Harper M.T. Lipid rafts are essential for release of phosphatidylserine-exposing extracellular vesicles from platelets. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-28363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connor D.E., Ly K., Aslam A., Boland J., Low J., Jarvis S., Muller D.W., Joseph J.E. Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets. 2016;27:805–811. doi: 10.1080/09537104.2016.1190008. [DOI] [PubMed] [Google Scholar]
- 40.Berckmans R.J., Lacroix R., Hau C.M., Sturk A., Nieuwland R. Extracellular vesicles and coagulation in blood from healthy humans revisited. J. Extracell. Vesicles. 2019;8 doi: 10.1080/20013078.2019.1688936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez E., Srivastava A.K., Burchfield J., Wang Y.W., Cardenas J.C., Togarrati P.P., Miyazawa B., Gonzalez E., Holcomb J.B., Pati S., et al. Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-53724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasecka A., Nieuwland R., Siljander P.R.M. Platelets. Elsevier; Amsterdam, The Netherlands: 2019. Platelet-derived extracellular vesicles; pp. 401–416. [Google Scholar]
- 43.French S.L., Butov K.R., Allaeys I., Canas J., Morad G., Davenport P., Laroche A., Trubina N.M., Italiano J.E., Moses M.A., et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4:3011–3023. doi: 10.1182/bloodadvances.2020001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tissot J.D., Canellini G., Rubin O., Angelillo-Scherrer A., Delobel J., Prudent M., Lion N. Blood microvesicles: From proteomics to physiology. Transl. Proteomics. 2013;1:38–52. doi: 10.1016/j.trprot.2013.04.004. [DOI] [Google Scholar]
- 45.Harisa G.I., Badran M.M., Alanazi F.K. Erythrocyte nanovesicles: Biogenesis, biological roles and therapeutic approach: Erythrocyte nanovesicles. Saudi Pharm. J. 2017;25:8–17. doi: 10.1016/j.jsps.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannopoulos G., Oudatzis G., Paterakis G., Synetos A., Tampaki E., Bouras G., Hahalis G., Alexopoulos D., Tousoulis D., Cleman M.W., et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014;176:145–150. doi: 10.1016/j.ijcard.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Mayr M., Grainger D., Mayr U., Leroyer A.S., Leseche G., Sidibe A., Herbin O., Yin X., Gomes A., Madhu B., et al. Proteomics, Metabolomics, and Immunomics on Microparticles Derived From Human Atherosclerotic Plaques. Circ. Cardiovasc. Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 48.Li K.Y., Zheng L., Wang Q., Hu Y.W. Characteristics of erythrocyte-derived microvesicles and its relation with atherosclerosis. Atherosclerosis. 2016;255:140–144. doi: 10.1016/j.atherosclerosis.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 49.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 2012;110:356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 50.La Salvia S., Musante L., Lannigan J., Gigliotti J.C., Le T.H., Erdbrügger U. T cell-derived extracellular vesicles are elevated in essential HTN. Am. J. Physiol. Renal Physiol. 2020;319:F868–F875. doi: 10.1152/ajprenal.00433.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zu L., Ren C., Pan B., Zhou B., Zhou E., Niu C., Wang X., Zhao M., Gao W., Guo L., et al. Endothelial microparticles after antihypertensive and lipid-lowering therapy inhibit the adhesion of monocytes to endothelial cells. Int. J. Cardiol. 2016;202:756–759. doi: 10.1016/j.ijcard.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 52.Melnikov I., Kozlov S., Saburova O., Zubkova E., Guseva O., Domogatsky S., Arefieva T., Radyukhina N., Zvereva M., Avtaeva Y., et al. Crp is transported by monocytes and monocyte-derived exosomes in the blood of patients with coronary artery disease. Biomedicines. 2020;8:435. doi: 10.3390/biomedicines8100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chistiakov D.A., Orekhov A.N., Bobryshevy Y.V. Cardiac extracellular vesicles in normal and infarcted heart. Int. J. Mol. Sci. 2016;17:63. doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loyer X., Zlatanova I., Devue C., Yin M., Howangyin K.Y., Klaihmon P., Guerin C.L., Khelouf M., Vilar J., Zannis K., et al. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction short communication. Circ. Res. 2018;123:100–106. doi: 10.1161/CIRCRESAHA.117.311326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paone S., Baxter A.A., Hulett M.D., Poon I.K.H. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell. Mol. Life Sci. 2019;76:1093–1106. doi: 10.1007/s00018-018-2983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sansone R., Baaken M., Horn P., Schuler D., Westenfeld R., Amabile N., Kelm M., Heiss C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Br. 2018;19:495–500. doi: 10.1016/j.dib.2018.04.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadid M., Lind J.U., Ardoña H.A.M., Sheehy S.P., Dickinson L.E., Eweje F., Bastings M.M.C., Pope B., O’Connor B.B., Straubhaar J.R., et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 2020;12:8005. doi: 10.1126/scitranslmed.aax8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berezin A.E., Berezin A.A. Extracellular Endothelial Cell-Derived Vesicles: Emerging Role in Cardiac and Vascular Remodeling in Heart Failure. Front. Cardiovasc. Med. 2020;7:47. doi: 10.3389/fcvm.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H., Wang Z. Cardiomyocyte-Derived Exosomes: Biological Functions and Potential Therapeutic Implications. Front. Physiol. 2019;10:1–9. doi: 10.3389/fphys.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heallen T.R., Martin J.F. Heart repair via cardiomyocyte-secreted vesicles. Nat. Biomed. Eng. 2018;2:271–272. doi: 10.1038/s41551-018-0239-5. [DOI] [PubMed] [Google Scholar]
- 61.Ribeiro-Rodrigues T.M., Laundos T.L., Pereira-Carvalho R., Batista-Almeida D., Pereira R., Coelho-Santos V., Silva A.P., Fernandes R., Zuzarte M., Enguita F.J., et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc. Res. 2017;113:1338–1350. doi: 10.1093/cvr/cvx118. [DOI] [PubMed] [Google Scholar]
- 62.Dougherty J.A., Kumar N., Noor M., Angelos M.G., Khan M., Chen C.-A., Khan M. Extracellular Vesicles Released by Human Induced-Pluripotent Stem Cell-Derived Cardiomyocytes Promote Angiogenesis. Front. Physiol. 2018;9:1–14. doi: 10.3389/fphys.2018.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J., Xue F., Li Y., Liu W., Zhang S., Yu X. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am. J. Transl. Res. 2018;10:4350–4366. [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B., Lee B.W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K., et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018;2:293–303. doi: 10.1038/s41551-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong S.G., Lee W.H., Zhou Y., Wu J.C. Methods in Molecular Biology. Vol. 1733. Humana Press Inc.; Totowa, NJ, USA: 2018. Mining exosomal MicroRNAs from human-induced pluripotent stem cells-derived cardiomyocytes for cardiac regeneration; pp. 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borosch S., Dahmen E., Beckers C., Stoppe C., Buhl E.M., Denecke B., Goetzenich A., Kraemer S. Characterization of extracellular vesicles derived from cardiac cells in an in vitro model of preconditioning. J. Extracell. Vesicles. 2017;6:1390391. doi: 10.1080/20013078.2017.1390391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren X.S., Tong Y., Qiu Y., Ye C., Wu N., Xiong X.Q., Wang J.J., Han Y., Zhou Y.B., Zhang F., et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles. 2020;9:1698795. doi: 10.1080/20013078.2019.1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakhshian Nik A., Hutcheson J.D., Aikawa E. Extracellular Vesicles As Mediators of Cardiovascular Calcification. Front. Cardiovasc. Med. 2017;4:1. doi: 10.3389/fcvm.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapustin A.N., Davies J.D., Reynolds J.L., McNair R., Jones G.T., Sidibe A., Schurgers L.J., Skepper J.N., Proudfoot D., Mayr M., et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 2011;109 doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 70.Furmanik M., Chatrou M., Van Gorp R., Akbulut A., Willems B., Schmidt H., Van Eys G., Bochaton-Piallat M.L., Proudfoot D., Biessen E., et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020;127:911–927. doi: 10.1161/CIRCRESAHA.119.316159. [DOI] [PubMed] [Google Scholar]
- 71.Petsophonsakul P., Furmanik M., Forsythe R., Dweck M., Schurink G.W., Natour E., Reutelingsperger C., Jacobs M., Mees B., Schurgers L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation involvement of Vitamin K-dependent processes. Arterioscler. Thromb. Vasc. Biol. 2019;39:1351–1368. doi: 10.1161/ATVBAHA.119.312787. [DOI] [PubMed] [Google Scholar]
- 72.Kapustin A.N., Shanahan C.M. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J. Physiol. 2016;594:2905–2914. doi: 10.1113/JP271340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Gonzalo-Calvo D., Cenarro A., Garlaschelli K., Pellegatta F., Vilades D., Nasarre L., Camino-Lopez S., Crespo J., Carreras F., Leta R., et al. Translating the microRNA signature of microvesicles derived from human coronary artery smooth muscle cells in patients with familial hypercholesterolemia and coronary artery disease. J. Mol. Cell. Cardiol. 2017;106:55–67. doi: 10.1016/j.yjmcc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y., Bao S., Guo W., Diao Z., Wang L., Han X., Guo W., Liu W. Bone marrow mesenchymal stem cell–derived exosomes alleviate high phosphorus-induced vascular smooth muscle cells calcification by modifying microRNA profiles. Funct. Integr. Genomics. 2019;19:633–643. doi: 10.1007/s10142-019-00669-0. [DOI] [PubMed] [Google Scholar]
- 75.Vajen T., Benedikter B.J., Heinzmann A.C.A., Vasina E.M., Henskens Y., Parsons M., Maguire P.B., Stassen F.R., Heemskerk J.W.M., Schurgers L.J., et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles. 2017;6:1322454. doi: 10.1080/20013078.2017.1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss R., Gröger M., Rauscher S., Fendl B., Eichhorn T., Fischer M.B., Spittler A., Weber V. Differential Interaction of Platelet-Derived Extracellular Vesicles with Leukocyte Subsets in Human Whole Blood. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-25047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Njock M.S., Cheng H.S., Dang L.T., Nazari-Jahantigh M., Lau A.C., Boudreau E., Roufaiel M., Cybulsky M.I., Schober A., Fish J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125:3202–3212. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosseinkhani B., Kuypers S., van den Akker N.M.S., Molin D.G.M., Michiels L. Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front. Immunol. 2018;9:6. doi: 10.3389/fimmu.2018.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niu C., Wang X., Zhao M., Cai T., Liu P., Li J., Willard B., Zu L., Zhou E., Li Y., et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyer M.J., Kimura Y., Akiyama T., Baggett A.Y., Preston K.J., Scalia R., Eguchi S., Rizzo V. Endothelial cell-derived extracellular vesicles alter vascular smooth muscle cell phenotype through high-mobility group box proteins. J. Extracell. Vesicles. 2020;9:1781427. doi: 10.1080/20013078.2020.1781427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song T., Lv M., Sun B., Zheng L., Zhao M. Tripeptides Val-Pro-Pro (VPP) and Ile-Pro-Pro (IPP) Regulate the Proliferation and Migration of Vascular Smooth Muscle Cells by Interfering Ang II-Induced Human Umbilical Vein Endothelial Cells Derived EVs Delivering RNAs to VSMCs in the Co-culture Model. J. Agric. Food Chem. 2020;68:6628–6637. doi: 10.1021/acs.jafc.0c02060. [DOI] [PubMed] [Google Scholar]
- 82.Wang H., Yan H.M., Tang M.X., Wang Z.H., Zhong M., Zhang Y., Deng J.T., Zhang W. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determinated by ELISA using a specific monoclonal antibody AD-1. Clin. Chim. Acta. 2010;411:1700–1704. doi: 10.1016/j.cca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Kulshreshtha A., Singh S., Ahmad M., Khanna K., Ahmad T., Agrawal A., Ghosh B. Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-52765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davidson S.M., Andreadou I., Barile L., Birnbaum Y., Cabrera-Fuentes H.A., Cohen M.V., Downey J.M., Girao H., Pagliaro P., Penna C., et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019;115:1156–1166. doi: 10.1093/cvr/cvy314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rautou P.E., Vion A.C., Amabile N., Chironi G., Simon A., Tedgui A., Boulanger C.M. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 86.Berezin A., Zulli A., Kerrigan S., Petrovic D., Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015;48:562–568. doi: 10.1016/j.clinbiochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Biasucci L.M., Porto I., di Vito L., de Maria G.L., Leone A.M., Tinelli G., Tritarelli A., di Rocco G., Snider F., Capogrossi M.C., et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 2012;76:2174–2182. doi: 10.1253/circj.CJ-12-0068. [DOI] [PubMed] [Google Scholar]
- 88.Chiva-Blanch G., Laake K., Myhre P., Bratseth V., Arnesen H., Solheim S., Badimon L., Seljeflot I. Platelet-, monocyte-derived & tissue factorcarrying circulating microparticles are related to acute myocardial infarction severity. PLoS ONE. 2017;12:2558. doi: 10.1371/journal.pone.0172558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mörtberg J., Lundwall K., Mobarrez F., Wallén H., Jacobson S.H., Spaak J. Increased concentrations of platelet- and endothelial-derived microparticles in patients with myocardial infarction and reduced renal function- a descriptive study. BMC Nephrol. 2019;20:71. doi: 10.1186/s12882-019-1261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suades R., Padró T., Crespo J., Ramaiola I., Martin-Yuste V., Sabaté M., Sans-Roselló J., Sionis A., Badimon L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016;202:378–387. doi: 10.1016/j.ijcard.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Burrello J., Bolis S., Balbi C., Burrello A., Provasi E., Caporali E., Gauthier L.G., Peirone A., D’Ascenzo F., Monticone S., et al. An extracellular vesicle epitope profile is associated with acute myocardial infarction. J. Cell. Mol. Med. 2020;24:9945–9957. doi: 10.1111/jcmm.15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deddens J.C., Vrijsen K.R., Colijn J.M., Oerlemans M.I., Metz C.H.G., van der Vlist E.J., Nolte-’t Hoen E.N.M., den Ouden K., Jansen Of Lorkeers S.J., van der Spoel T.I.G., et al. Circulating Extracellular Vesicles Contain miRNAs and are Released as Early Biomarkers for Cardiac Injury. J. Cardiovasc. Transl. Res. 2016;9:291–301. doi: 10.1007/s12265-016-9705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ge X., Meng Q., Zhuang R., Yuan D., Liu J., Lin F., Fan H., Zhou X. Circular RNA expression alterations in extracellular vesicles isolated from murine heart post ischemia/reperfusion injury. Int. J. Cardiol. 2019;296:136–140. doi: 10.1016/j.ijcard.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 94.Thulin Å., Lindbäck J., Granger C.B., Wallentin L., Lind L., Siegbahn A. Extracellular vesicles in atrial fibrillation and stroke. Thromb. Res. 2020;193:180–189. doi: 10.1016/j.thromres.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 95.Nozaki T., Sugiyama S., Sugamura K., Ohba K., Matsuzawa Y., Konishi M., Matsubara J., Akiyama E., Sumida H., Matsui K., et al. Prognostic value of endothelial microparticles in patients with heart failure. Eur. J. Heart Fail. 2010;12:1223–1228. doi: 10.1093/eurjhf/hfq145. [DOI] [PubMed] [Google Scholar]
- 96.Berezin A.E., Kremzer A.A., Samura T.A., Martovitskaya Y.V. Circulating endothelial-derived apoptotic microparticles in the patients with ischemic symptomatic chronic heart failure: Relevance of pro-inflammatory activation and outcomes. Int. Cardiovasc. Res. J. 2014;8:116–123. [PMC free article] [PubMed] [Google Scholar]
- 97.Yang J., Xue F.T., Li Y.Y., Liu W., Zhang S. Exosomal piRNA sequencing reveals differences between heart failure and healthy patients. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7952–7961. doi: 10.26355/eurrev-201811-16423. [DOI] [PubMed] [Google Scholar]
- 98.Perdomo L., Vidal-Gómez X., Soleti R., Vergori L., Duluc L., Chwastyniak M., Bisserier M., Le Lay S., Villard A., Simard G., et al. Large extracellular vesicle-associated rap1 accumulates in atherosclerotic plaques, correlates with vascular risks and is involved in atherosclerosis. Circ. Res. 2020:747–760. doi: 10.1161/CIRCRESAHA.120.317086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng B., Chen Y., Luo Y., Chen M., Li X., Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–269. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 100.Suades R., Padró T., Alonso R., López-Miranda J., Mata P., Badimon L. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb. Haemost. 2013;111:111–121. doi: 10.1160/TH13-07-0612. [DOI] [PubMed] [Google Scholar]
- 101.Kenneweg F., Bang C., Xiao K., Boulanger C.M., Loyer X., Mazlan S., Schroen B., Hermans-Beijnsberger S., Foinquinos A., Hirt M.N., et al. Long Noncoding RNA-Enriched Vesicles Secreted by Hypoxic Cardiomyocytes Drive Cardiac Fibrosis. Mol. Ther. Nucleic Acids. 2019;18:363–374. doi: 10.1016/j.omtn.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jansen F., Yang X., Proebsting S., Hoelscher M., Przybilla D., Baumann K., Schmitz T., Dolf A., Endl E., Franklin B.S., et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen S., Shiesh S.C., Lee G.B., Chen C. Two-step magnetic bead-based (2MBB) techniques for immunocapture of extracellular vesicles and quantification of microRNAs for cardiovascular diseases: A pilot study. PLoS ONE. 2020;15:e0229610. doi: 10.1371/journal.pone.0229610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Z., Zhang J., Zhang S., Yan S., Wang Z., Wang C., Zhang X. MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1. Mol. Med. Rep. 2019;19:3298–3304. doi: 10.3892/mmr.2019.9983. [DOI] [PubMed] [Google Scholar]
- 105.Bi S., Wang C., Jin Y., Lv Z., Xing X., Lu Q. Correlation between serum exosome derived miR-208A and acute coronary syndrome. Int. J. Clin. Exp. Med. 2015;8:4275–4280. [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumoto S., Sakata Y., Suna S., Nakatani D., Usami M., Hara M., Kitamura T., Hamasaki T., Nanto S., Kawahara Y., et al. Circulating p53-responsive MicroRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 107.Wu T., Chen Y., Du Y., Tao J., Zhou Z., Yang Z. Serum Exosomal MiR-92b-5p as a Potential Biomarker for Acute Heart Failure Caused by Dilated Cardiomyopathy. Cell. Physiol. Biochem. 2018;46:1939–1950. doi: 10.1159/000489383. [DOI] [PubMed] [Google Scholar]
- 108.Fitzsimons S., Oggero S., Bruen R., McCarthy C., Strowitzki M.J., Mahon N.G., Ryan N., Brennan E.P., Barry M., Perretti M., et al. microRNA-155 Is Decreased During Atherosclerosis Regression and Is Increased in Urinary Extracellular Vesicles During Atherosclerosis Progression. Front. Immunol. 2020;11:1. doi: 10.3389/fimmu.2020.576516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Li Q., Hosen M.R., Zietzer A., Flender A., Levermann P., Schmitz T., Frühwald D., Goody P., Nickenig G., et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ. Res. 2019;124:575–587. doi: 10.1161/CIRCRESAHA.118.314010. [DOI] [PubMed] [Google Scholar]
- 110.Chang Y.J., Li Y.S., Wu C.C., Wang K.C., Huang T.C., Chen Z., Chien S. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019;39:2492–2504. doi: 10.1161/ATVBAHA.119.312707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nguyen M.A., Karunakaran D., Geoffrion M., Cheng H.S., Tandoc K., Perisic Matic L., Hedin U., Maegdefessel L., Fish J.E., Rayner K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2018;38:49–63. doi: 10.1161/ATVBAHA.117.309795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinning J.M., Losch J., Walenta K., Böhm M., Nickenig G., Werner N. Circulating CD31+/Annexin V + microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011;32:2034–2041. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 113.Cheow E.S.H., Cheng W.C., Lee C.N., De Kleijn D., Sorokin V., Sze S.K. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for Myocardial Ischemic (MI) injury. Mol. Cell. Proteomics. 2016;15:2628–2640. doi: 10.1074/mcp.M115.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nozaki T., Sugiyama S., Koga H., Sugamura K., Ohba K., Matsuzawa Y., Sumida H., Matsui K., Jinnouchi H., Ogawa H. Significance of a Multiple Biomarkers Strategy Including Endothelial Dysfunction to Improve Risk Stratification for Cardiovascular Events in Patients at High Risk for Coronary Heart Disease. J. Am. Coll. Cardiol. 2009;54:601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 115.Dekker M., Waissi F., van Bennekom J., Silvis M.J.M., Timmerman N., Bank I.E.M., Walter J.E., Mueller C., Schoneveld A.H., Schiffelers R.M., et al. Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-69297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Habersberger J., Strang F., Scheichl A., Htun N., Bassler N., Merivirta R.M., Diehl P., Krippner G., Meikle P., Eisenhardt S.U., et al. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovasc. Res. 2012;96:64–72. doi: 10.1093/cvr/cvs237. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y.N., Vernooij F., Ibrahim I., Ooi S., Gijsberts C.M., Schoneveld A.H., Sen K.W., Den Ruijter H.M., Timmers L., Richards A.M., et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: An observational study in a dyspnoea cohort. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0148073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Hoog V.C., Timmers L., Schoneveld A.H., Wang J.W., Van de Weg S.M., Sze S.K., Van Keulen J.K., Hoes A.W., Den Ruijter H.M., de Kleijn D.P., et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Hear. J. Acute Cardiovasc. Care. 2013;2:53–60. doi: 10.1177/2048872612471212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crawford J.R., Trial J.A., Nambi V., Hoogeveen R.C., Taffet G.E., Entman M.L. Plasma Levels of Endothelial Microparticles Bearing Monomeric C-reactive Protein are Increased in Peripheral Artery Disease. J. Cardiovasc. Transl. Res. 2016;9:184–193. doi: 10.1007/s12265-016-9678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rogers M.A., Buffolo F., Schlotter F., Atkins S.K., Lee L.H., Halu A., Blaser M.C., Tsolaki E., Higashi H., Luther K., et al. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci. Adv. 2020;6:eabb1244. doi: 10.1126/sciadv.abb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwon S.H., Woollard J.R., Saad A., Garovic V.D., Zand L., Jordan K.L., Textor S.C., Lerman L.O. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol. Dial. Transplant. 2017;32:800–807. doi: 10.1093/ndt/gfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santelli A., Sun I.O., Eirin A., Abumoawad A.M., Woollard J.R., Lerman A., Textor S.C., Puranik A.S., Lerman L.O. Senescent Kidney Cells in Hypertensive Patients Release Urinary Extracellular Vesicles. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agudiez M., Martinez P.J., Martin-Lorenzo M., Heredero A., Santiago-Hernandez A., Molero D., Garcia-Segura J.M., Aldamiz-Echevarria G., Alvarez-Llamas G. Analysis of urinary exosomal metabolites identifies cardiovascular risk signatures with added value to urine analysis. BMC Biol. 2020;18:192. doi: 10.1186/s12915-020-00924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burrello J., Biemmi V., Dei Cas M., Amongero M., Bolis S., Lazzarini E., Bollini S., Vassalli G., Paroni R., Barile L. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-73411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peng M., Liu X., Xu G. Extracellular Vesicles as Messengers in Atherosclerosis. J. Cardiovasc. Transl. Res. 2020;13:121–130. doi: 10.1007/s12265-019-09923-z. [DOI] [PubMed] [Google Scholar]
- 126.Peters L.J.F., Biessen E.A.L., Hohl M., Weber C., van der Vorst E.P.C., Santovito D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020;11:793. doi: 10.3389/fphys.2020.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 128.Pritchard C.C., Cheng H.H., Tewari M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saliminejad K., Khorram Khorshid H.R., Soleymani Fard S., Ghaffari S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 130.Rayner K.J., Hennessy E.J. Extracellular communication via microRNA: Lipid particles have a new message. J. Lipid Res. 2013;54:1174–1181. doi: 10.1194/jlr.R034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H., Peng R., Wang J., Qin Z., Xue L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenet. 2018;10:1–10. doi: 10.1186/s13148-018-0492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu X., Odenthal M., Fries J. Exosomes as miRNA Carriers: Formation–Function–Future. Int. J. Mol. Sci. 2016;17:2028. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng H.L., Fu C.Y., Kuo W.C., Chen Y.W., Chen Y.S., Lee Y.M., Li K.H., Chen C., Ma H.P., Huang P.C., et al. Detecting miRNA biomarkers from extracellular vesicles for cardiovascular disease with a microfluidic system. Lab Chip. 2018;18:2917–2925. doi: 10.1039/C8LC00386F. [DOI] [PubMed] [Google Scholar]
- 134.Rogers M.A., Aikawa E. MicroRNA Extracellular Vesicle Stowaways in Cell-Cell Communication and Organ Crosstalk. Arterioscler. Thromb. Vasc. Biol. 2019;39:2448–2450. doi: 10.1161/ATVBAHA.119.313533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang F., Chen Q., Wang W., Ling Y., Yan Y., Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2019;72:156–166. doi: 10.1016/j.jhep.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 136.Wang X., Huang W., Liu G., Cai W., Millard R.W., Wang Y., Chang J., Peng T., Fan G.C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y., Meng Y., Zhu X., Saadiq I.M., Jordan K.L., Eirin A., Lerman L.O. Metabolic syndrome increases senescence-associated micro-RNAs in extracellular vesicles derived from swine and human mesenchymal stem/stromal cells. Cell Commun. Signal. 2020;18:124. doi: 10.1186/s12964-020-00624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mallia A., Gianazza E., Zoanni B., Brioschi M., Barbieri S.S., Banfi C. Proteomics of extracellular vesicles: Update on their composition, biological roles and potential use as diagnostic tools in atherosclerotic cardiovascular diseases. Diagnostics. 2020;10:843. doi: 10.3390/diagnostics10100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu R., Gao W., Yao K., Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front. Immunol. 2019;10:648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J.W., Gijsberts C.M., Seneviratna A., De Hoog V.C., Vrijenhoek J.E.P., Schoneveld A.H., Chan M.Y., Lam C.S.P., Richards A.M., Lee C.N., et al. Plasma extra cellular vesicle protein content for diagnosis and prognosis of global cardiovascular disease. Neth. Hear. J. 2013;21:467–471. doi: 10.1007/s12471-013-0462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 142.Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:1–60. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arvidson G., Ronquist G., Wikander G., Öjteg A.C. Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. BBA Biomembr. 1989;984:167–173. doi: 10.1016/0005-2736(89)90212-5. [DOI] [PubMed] [Google Scholar]
- 144.Kalra H., Simpson R.J., Ji H., Aikawa E., Altevogt P., Askenase P., Bond V.C., Borràs F.E., Breakefield X., Budnik V., et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim D.K., Kang B., Kim O.Y., Choi D.S., Lee J., Kim S.R., Go G., Yoon Y.J., Kim J.H., Jang S.C., et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simpson R.J., Kalra H., Mathivanan S. Exocarta as a resource for exosomal research. J. Extracell. Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Keyel P.A., Tkacheva O.A., Larregina A.T., Salter R.D. Coordinate Stimulation of Macrophages by Microparticles and TLR Ligands Induces Foam Cell Formation. J. Immunol. 2012;189:4621–4629. doi: 10.4049/jimmunol.1200828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heemskerk J.W.M., Mattheij N.J.A., Cosemans J.M.E.M. Platelet-based coagulation: Different populations, different functions. J. Thromb. Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 149.Zarà M., Guidetti G.F., Camera M., Canobbio I., Amadio P., Torti M., Tremoli E., Barbieri S.S. Biology and role of extracellular vesicles (Evs) in the pathogenesis of thrombosis. Int. J. Mol. Sci. 2019;20:2840. doi: 10.3390/ijms20112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018;59:2037–2046. doi: 10.1194/jlr.R084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suades R., Padró T., Alonso R., Mata P., Badimon L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb. Haemost. 2013;110:366–377. doi: 10.1160/TH13-03-0238. [DOI] [PubMed] [Google Scholar]
- 152.Mobarrez F., Egberg N., Antovic J., Bröijersen A., Jörneskog G., Wallén H. Release of endothelial microparticles in vivo during atorvastatin treatment; A randomized double-blind placebo-controlled study. Thromb. Res. 2012;129:95–97. doi: 10.1016/j.thromres.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 153.Gasecka A., Nieuwland R., Budnik M., Dignat-George F., Eyileten C., Harrison P., Lacroix R., Leroyer A., Opolski G., Pluta K., et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J. Thromb. Haemost. 2020;18:609–623. doi: 10.1111/jth.14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chyrchel B., Drożdż A., Długosz D., Stȩpień E., Surdacki A. Platelet reactivity and circulating platelet-derived microvesicles are differently affected by P2Y 12 receptor antagonists. Int. J. Med. Sci. 2019;16:264–275. doi: 10.7150/ijms.28580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burnley-Hall N., Abdul F., Androshchuk V., Morris K., Ossei-Gerning N., Anderson R., Rees D.A., James P.E. Dietary Nitrate Supplementation Reduces Circulating Platelet-Derived Extracellular Vesicles in Coronary Artery Disease Patients on Clopidogrel Therapy: A Randomised, Double-Blind, Placebo-Controlled Study. Thromb. Haemost. 2018;118:112–122. doi: 10.1160/TH17-06-0394. [DOI] [PubMed] [Google Scholar]
- 156.Lu M., Xing H., Yang Z., Sun Y., Yang T., Zhao X., Cai C., Wang D., Ding P. Recent advances on extracellular vesicles in therapeutic delivery: Challenges, solutions, and opportunities. Eur. J. Pharm. Biopharm. 2017;119:381–395. doi: 10.1016/j.ejpb.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 158.De Jong O.G., Kooijmans S.A.A., Murphy D.E., Jiang L., Evers M.J.W., Sluijter J.P.G., Vader P., Schiffelers R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019;52:1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davidson S.M., Takov K., Yellon D.M., Davidson S.M. Exosomes and Cardiovascular Protection. Cardiovasc. Drugs Ther. 2017:77–86. doi: 10.1007/s10557-016-6698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ong S., Lee W.H., Huang M., Dey D., Kodo K., Sanchez-freire V., Gold J.D., Wu J.C. Cross Talk of Combined Gene and Cell Therapy in Ischemic Heart Disease—Role of Exosomal MicroRNA Transfer. Circulation. 2016;130:1–20. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zheng B., Yin W.N., Suzuki T., Zhang X.H., Zhang Y., Song L.L., Jin L.S., Zhan H., Zhang H., Li J.S., et al. Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol. Ther. 2017;25:1279–1294. doi: 10.1016/j.ymthe.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.Song Y., Zhang C., Zhang J., Jiao Z., Dong N., Wang G., Wang Z., Wang L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. 2019;9:2346–2360. doi: 10.7150/thno.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo Z., Zhao Z., Yang C., Song C. Transfer of microRNA-221 from mesenchymal stem cell-derived extracellular vesicles inhibits atherosclerotic plaque formation. Transl. Res. 2020;226:83–95. doi: 10.1016/j.trsl.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 164.Vicencio J.M., Yellon D.M., Sivaraman V., Das D., Boi-Doku C., Arjun S., Zheng Y., Riquelme J.A., Kearney J., Sharma V., et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 165.Van Hezel M.E., Nieuwland R., van Bruggen R., Juffermans N.P. The ability of extracellular vesicles to induce a pro-inflammatory host response. Int. J. Mol. Sci. 2017;18:1285. doi: 10.3390/ijms18061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma Q., Fan Q., Han X., Dong Z., Xu J., Bai J., Tao W., Sun D., Wang C. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Release. 2021;329:445–453. doi: 10.1016/j.jconrel.2020.11.064. [DOI] [PubMed] [Google Scholar]
- 167.Wu G., Zhang J., Zhao Q., Zhuang W., Ding J., Zhang C., Gao H., Pang D., Pu K., Xie H. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chem. 2020;132:4097–4103. doi: 10.1002/ange.201913700. [DOI] [PubMed] [Google Scholar]
- 168.Ban J.J., Lee M., Im W., Kim M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015;461:76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 169.Maroto R., Zhao Y., Jamaluddin M., Popov V.L., Wang H., Kalubowilage M., Zhang Y., Luisi J., Sun H., Culbertson C.T., et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles. 2017;6 doi: 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J., Nilsson J., Lötvall J., Kim Y.K., Gho Y.S. Erratum: Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors (ACS Nano (2013) 7 (7698–7710) doi:10.1021/nn402232g) ACS Nano. 2014;8:1073. doi: 10.1021/nn406580h. [DOI] [PubMed] [Google Scholar]
- 171.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 172.Gangadaran P., Ahn B.C. Extracellular vesicle-and extracellular vesicle mimetics-based drug delivery systems: New perspectives, challenges, and clinical developments. Pharmaceutics. 2020;12:442. doi: 10.3390/pharmaceutics12050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu M., Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242:119925. doi: 10.1016/j.biomaterials.2020.119925. [DOI] [PubMed] [Google Scholar]
- 174.Costa Verdera H., Gitz-Francois J.J., Schiffelers R.M., Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 175.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 176.Cully M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2020;20:6–7. doi: 10.1038/d41573-020-00220-y. [DOI] [PubMed] [Google Scholar]