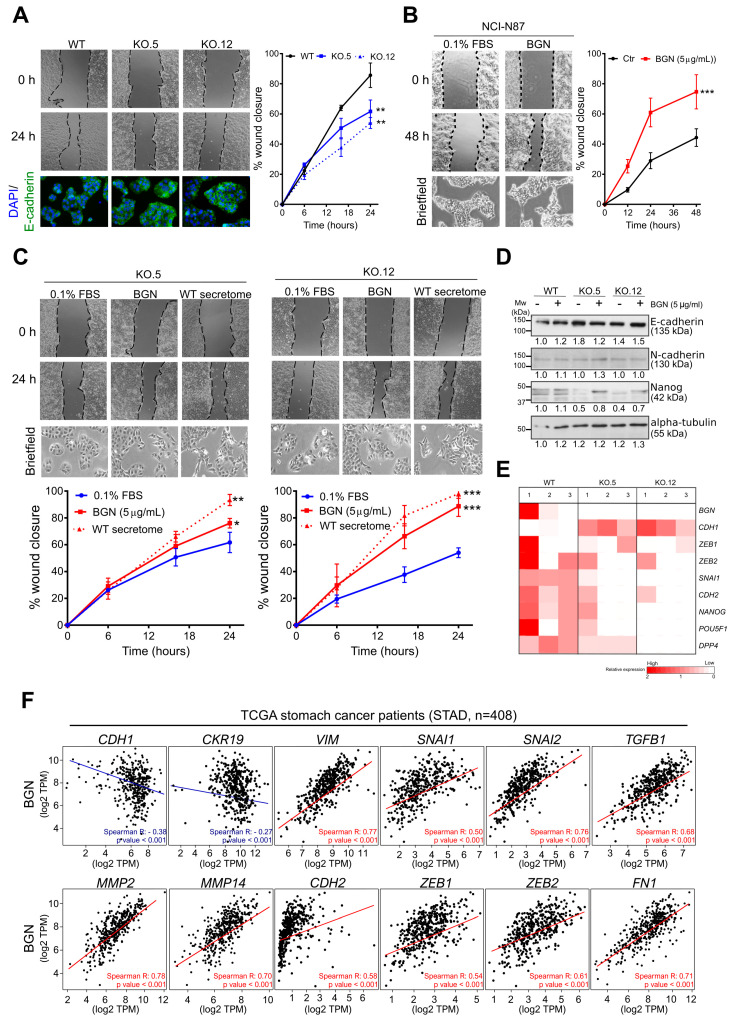
Figure 4.
Biglycan modulates GC cell migration through the regulation of epithelial-mesenchymal (EMT) markers. (A) Wound-healing migration assay in MKN74 WT and in biglycan KO cells. The loss of biglycan decreases cell migration capacity with a concomitant increase of E-cadherin (bottom images, magnification at 200×). (B) Treatment with 5 μg/mL of commercial purified biglycan is able to increase cell migration in the NCI-N87 biglycan-negative cell line. Exogenous biglycan is also able to promote a mesenchymal-like phenotype with more elongated spindle-like cells. Images magnification at 200× (C) Migration capacity of biglycan KO cells upon exogenous treatment with commercial purified biglycan or secretome from MKN74 WT (biglycan positive). Both treatments are able to restore migration capacity of KO cells and to promote a mesenchymal-like phenotype of cells. Images magnification at 200× (D) Western blot analysis of epithelial (E-cadherin) and mesenchymal (N-cadherin and Nanog) markers with or without biglycan treatment. (E) Expression levels of epithelial markers (CDH1 or E-cadherin) as well as mesenchymal markers (CDH2 or N-cadherin, SNAI1, ZEB1, ZEB2, NANOG, POU5F1, and DPP4) were examined by qRT-PCR. Biglycan KO cells present increased expression of CDH1 and decreased expression in mesenchymal markers such as CDH2, SNAI1, and ZEB2. Three independent analysis were performed in duplicate. (F) In silico analysis of epithelial and mesenchymal genes in human stomach cancer samples (STAD-TCGA, n = 408) using the Gene Expression Profiling Interactive Analysis (GEPIA) program corroborating the in vitro findings. In vitro data are presented as the mean ± standard error of the mean (S.E.M.) of at least three independent experiments. *, p value < 0.05; **, p value < 0.01; ***, p value < 0.001.