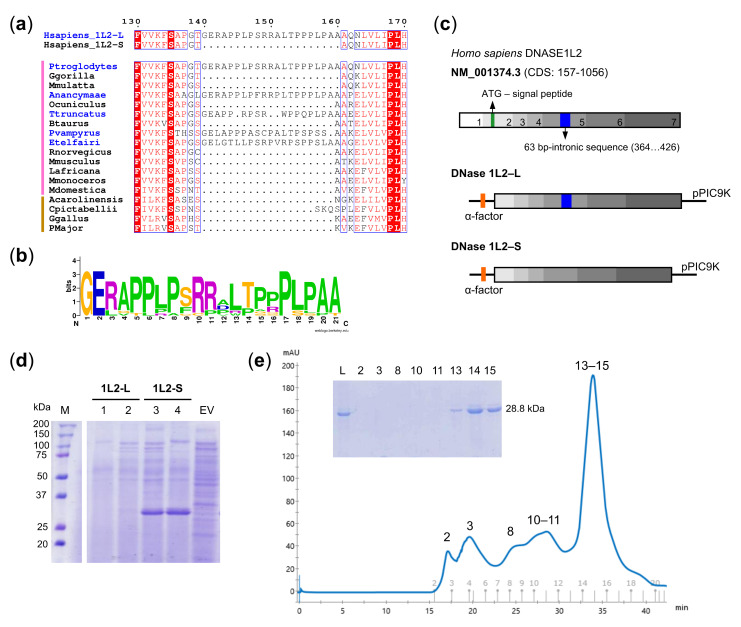
Figure 1.
Expression and purification of DNase1L2. (a) Portion of multiple alignment showing the proline-rich region present only in some mammalian DNase1L2 sequences (names in blue). Vertical lines on the left indicate sequence from mammals (pink) and sauropsids (brown). (b) Frequency plot of the aligned proline-rich regions from thirteen mammalian DNase1L2 sequences showing remarkable conservation. (c) Scheme of the DNase1L2 mRNA sequence (NM_001374.3) and the constructs designed for the expression of DNase1L2-L and DNase1L2-S in P. pastoris GS115. CDS lacking the predicted signal peptide were cloned into pPIC9K vector bearing a sequence (α-factor) for the secretion of expressed proteins. (d) SDS-PAGE analysis of extracellular expression of P. pastoris GS115 cells transformed with pPIC9K-DNase1L2-L (two clones, 1 and 2, are shown) or with pPIC9K-DNase1L2-S (two clones, 3 and 4, are shown) or with an empty vector (EV). The band corresponding to DNase1L2-S is found at the expected molecular weight of 28.8 kDa. M: Marker. (e) Size-exclusion chromatogram and SDS-PAGE (inset) showing the final step of DNase1L2 purification. Numerals above peaks correspond to the gel lanes in the inset. L: Load.