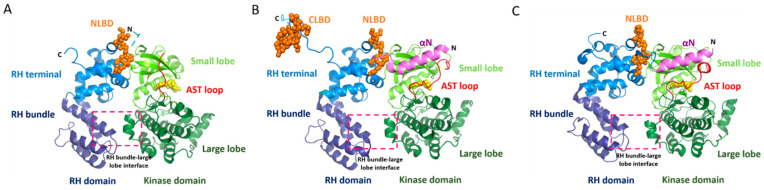
Figure 1.
Crystal structures representing inactive and active conformations of the G protein-coupled receptors kinase 4 (GRK4) subfamily members. (A) Crystal structure of GRK6 in a complex with adenylyl imidodiphosphate (Protein Data Bank (PDB) entry: 2ACX). (B) Crystal structure of GRK6 in a complex with sangivamycin (PDB: 3NYN). (C) Crystal structure of GRK5 in complex with Ca2+·CaM (PDB: 6PJX), with Ca2+·CaM removed for the sake of comparison. The regulator of the G protein signaling homology (RH) and kinase domains are shown in blue and green, respectively. The active site tether (AST) loop and N-terminal (αN) helix (disordered in panel A) are highlighted in red and magenta, respectively. The N-terminal lipid-binding domain (NLBD) and C-terminal lipid-binding domain (CLBD) are shown with orange spheres in the structures where they were ordered. The RH bundle–large lobe interface is highlighted with a red dashed box. Ligands bound in the active site are shown with yellow spheres. Note the relative closure of the kinase domain in panels B and C relative to panel A, as well as the ordering of the αN helix and the AST region, which coalesce in the activated structures.