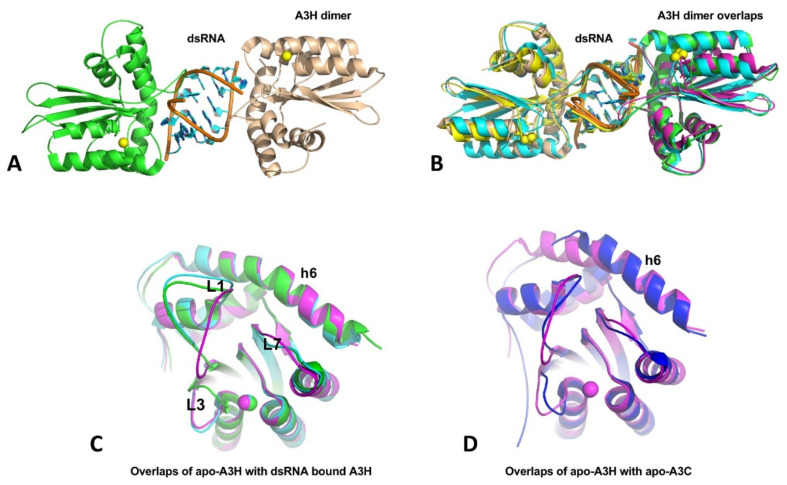
Figure 6.
The crystal structures of A3H and dimer formation through dsRNA binding. (A) The A3H dimer (5W3V) with two subunits being connected through a dsRNA bound in between. No protein-protein contact exists between the two subunits within a dimer. (B) Superimposition of three available A3H dimers from three different organisms (5W3V, 6BBO, 5Z98), showing highly conserved dimerization mechanisms. (C) Superimposition of apo-A3H monomer structure with one subunit from the A3H dimers (5W45, 5W3V, and 5Z98), showing that conformational differences only for loops 1, 3, and 7, which are directly involved in dsRNA binding. (D) Superimposition of apo-A3H (5W45) and apo-A3C (3VOW) reveal that A3H has two extra alpha-helical turns for h6, compared to A3C and all other known APOBEC structures.