Abstract
Invasive fungal infections represent an expanding threat to public health. During the past decade, a paradigm shift of candidiasis from Candida albicans to non-albicans Candida species has fundamentally increased with the advent of Candida auris. C. auris was identified in 2009 and is now recognized as an emerging species of concern and underscores the urgent need for novel drug development strategies. In this review, we discuss the genomic epidemiology and the main virulence factors of C. auris. We also focus on the different new strategies and results obtained during the past decade in the field of antifungal design against this emerging C. auris pathogen yeast, based on a medicinal chemist point of view. Critical analyses of chemical features and physicochemical descriptors will be carried out along with the description of reported strategies.
Keywords: Candida auris, antifungal drugs, repurposed drugs, combinatorial therapy, natural compounds, nanoparticles
1. Introduction
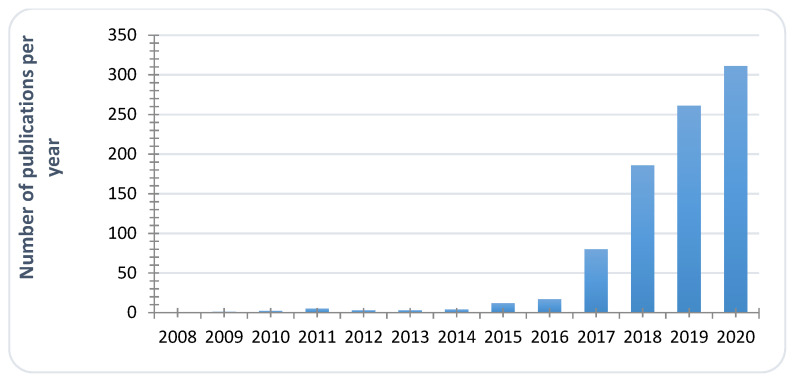
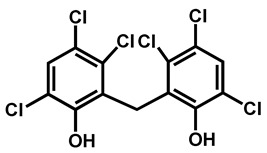
First identified in 2009 from the ear canal of a patient in Japan [1], C. auris is now recognized as an emerging species of concern [2,3], and more and more publications are related to C. auris studies (Figure 1). When searching for “Candida auris” key words on Scifinder, fewer than 1000 reports have been compiled, most of them during the two last years, proving the acceleration of research and clinical studies around this emerging pathogen. Its emergence is defined by the occurrence of C. auris infections in a dozen of countries all around the world. In Europe, since 2015, sporadic epidemics have been reported in Spain [4], United-Kingdom [5], Germany, or Norway [6], for example. Simultaneously, candidiasis caused by C. auris were reported in Korea [7], South Korea [8], India [9,10,11], South-Africa [12], or Kowait [13]. In the same period, the United States were also affected [14,15,16]. This human pathogen is associated with severe invasive infections with high mortality rates ranging from 35 to 72% [8,12,17,18]. Since June 2016, governmental institutions (Centers for Disease Control and Prevention (CDC), European Centre for Disease Prevention and Control (ECDC), World Health Organization (WHO), Pan American Health Organization (PAHO), National institute for Communicable Diseases (NICD)…) have issued clinical alerts to health care facilities and provided interim guidelines for clinical management, laboratory testing and infection control of C. auris [19,20]. This fungus poses significant challenges to microbiologists and clinicians because of its frequent multidrug resistance; high transmissibility and severe outcomes coupled with misidentification by standard biochemical identification systems such as Vitek 2 [21].
Figure 1.
Evolution of annual reports dealing with C. auris studies (Scifinder search, key word Candida auris, Data Compiled on 31 December 2020).
Moreover, the widespread and prolonged use of available antifungal drugs has given rise to multidrug resistance that alters the therapeutic available options. It has been reported that nearly 90% of C. auris isolates exhibit resistance to fluconazole, around 30% resistance to amphotericin B, and less than 5% resistance to echinocandins [22,23]. Under such compelling circumstances, C. auris displays all the features of a “superbug” and efforts are ongoing to identify new therapeutic alternatives to fight against C. auris infections. After introducing C. auris genomic epidemiology and virulence factors, this review aims at compiling the different strategies and results obtained during the past ten years in the field of antifungal research against emerging C. auris from a medicinal chemist point of view. To summarize, six complementary strategies have been developed, from the most attractive to the less reported: (i) repurposing of drugs; (ii) evaluation of combination drugs; (iii) discovery of new drug-candidates; (iv) traditional medicines and natural compounds; (v) metal, metalloids and complexes; (vi) others approaches including nanoparticles and irradiation. Results and data will be analyzed from a chemical approach based on chemical features and physicochemical descriptors such as lipophilicity (expressed by logP) and topological polar surface area (TPSA) in order to establish a comparison between classes of compounds and define potential common pharmacophoric moieties.
2. Candida auris Genomic Epidemiology and Virulence Factors
2.1. Global View
C. auris is taxonomically placed as a close relative to the Candida haemulonii since its discovery in 2009 from the ear canal of a patient in Japan [1]. C. auris was isolated in all continents except Antarctica. Munoz et al. found that four of the five clades of C. auris are genetically related to other Candida species including C. haemulonii, C. duobushaemulonii, and C. psuedohaemulonii [24]. These five genetically distinct clades of C. auris correspond to: Clade I from India and Pakistan (South Asian), Clade II from Japan (East Asian), Clade III from South Africa (African), Clade IV from Venezuela (South American) and most recently a potential Clade V from Iran [22,25]. Over the past ten years, C. auris has been isolated across all major continents, including elsewhere in Asia, Africa, North and South America, Australia, Europe, and the Middle East.
Outbreaks in United States have been reported to different clades of C. auris that are introduced from other continents. In terms of the analysis of whole-genome sequencing studies, Chow et al. found that the 133 clinical isolates of C. auris identified in United-States between 2013 and 2017 were related to South Asian, South American, African, and East Asian isolates but unexpectedly, only 7% of these clinical C. auris isolates were identified from patients with clear evidence of being acquired through health-care exposures abroad [16]. Outbreaks in Europe were also attributed to recent spread from other continents. For example, the first case of C. auris infection in France has been reported in 2018 from a patient who travelled in India and Iran two months before his hospitalization in France [26].
C. auris is believed to have been misidentified as C. haemulonii on several occasions, suggesting that C. auris has likely been circulating as a human fungal pathogen before 2009. However, retrospective analysis of the SENTRY Antifungal Surveillance collection of over 15,000 Candida isolates from 135 participating medical centers in North America, Europe, Latin America, and the Asia-Pacific regions since 1997 shows no misidentifications of C. auris before 2009 [27].
2.2. Virulence Factors of C. auris
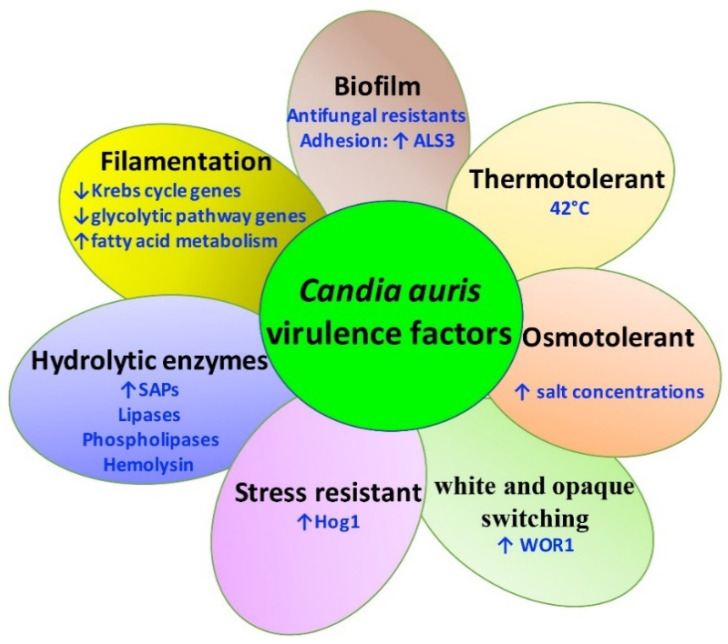
Like C. albicans, C. auris expresses several virulence factors that contribute to pathogenesis including the transition between blastoconidia and filamentous forms, hydrolytic enzyme production, thermotolerance, biofilm/adhesion to host cells, osmotolerance, filamentation, and phenotypic switching (Figure 2).
Figure 2.
Schematic overview of C. auris virulence factors. Stress resistance, hydrolytic enzyme production, thermotolerance, biofilm/adhesion to host cells, osmotolerance, filamentation, and white and opaque switching are important virulence traits in C. auris.
In contrast to other close relatives including C. haemulonii or C. pseudohaemulonii, C. auris is able to grow at 42 °C [1]. The thermotolerance of C. auris contributes to persist against the high fever response and causes an invasive candidemia [28]. Casadevall et al. suggested that the thermotolerance of C. auris is related to climate change and global temperature changes supporting the hypothesis that C. auris is the first example of a new pathogenic fungus emerging from human-induced global warming [29].
C. auris is also osmotolerant and known to survive at high salt concentrations, which might enable it to survive environmentally including hypersaline desert lakes, salt-evaporating ponds, or tidal pools (Figure 2) [28]. C. auris is able to survive on human skin and environmental surfaces for several weeks and can even tolerate being exposed to some commonly used disinfectants [30].
In terms of filamentation of C. auris, Yue et al. showed that C. auris can switch to three distinct cell types including typical yeast, filamentation-competent (FC) yeast, and a filamentous form [31]. Of note, typical yeast cells are filamentation-incompetent while FC yeast and filamentous cells are filamentation-competent under specific in vitro culture conditions [31]. Furthermore, the difference between the typical yeast and filamentous cell types was variable among the expression levels of metabolism genes. In particular, both Krebs cycle- and glycolytic pathway-associated genes were downregulated in filamentous C. auris cells while fatty acid metabolism-related genes were upregulated, supporting that C. auris employs different metabolic modes to adapt to its host to reap the overall benefits of its commensal and pathogenic lifestyle [31]. Additionally, some strains of C. auris do not produce pseudohypae and show a decreased ability to assimilate different carbon sources (galactose, l−sorbose, cellobiose, l−arabinose, ethanol, glycerol, salicin, or citrate) [1].
Another morphological switch implicates the interconversion between white and opaque forms of C. auris [32]. Opaque yeast cells are more frequent colonizers in skin infections than white cells, whereas white yeast cells are much virulent in systemic infections than opaque cells [33,34]. Bentz et al. showed that C. auris phenotypic switching is regulated by WOR1 that control phenotypic switching in C. auris [33].
Like C. albicans, C. auris can produce the extracellular hydrolytic enzymes that are recognized as an important virulence trait including proteinases, hemolysins, lipases and phospholipases. Regarding secreted aspartyl proteinases (SAPs), that are one of the most significant extracellular enzymes produced in Candida species [35], SAPs contribute to degradation of host tissue by providing nutrients for pathogen propagation and promote the inactivation of host antimicrobial peptides, the evasion of the immune responses and the induction of inflammatory mediator release from host cells [36]. In terms of the difference of SAP activity between C. auris and C. albicans, C. auris is able to maintain high SAP activity at 42 °C when compared to that of C. albicans supporting that C. auris maintains its pathogenicity at higher temperatures [36].
Another important group of lytic enzymes are the lipases and phospholipases that are involved in the host damage, immune evasion and biofilm formation [37,38]. In contrast to C. albicans, the production of C. auris lipases or phospholipases appears to be decreased and strain-dependent although C. auris and C. albicans share the same quantity of lipase encoding genes in the genome [39].
Hemolysin is an exotoxin that is capable of lysing red blood cells as well as nucleated cells and different pathogenic Candida species including C. auris display hemolysin activity [40]. C. auris strains isolated from hospital infections exhibit a high production of hemolysin when compared to those from environmental sources suggesting that hemolysin activity is involved in C. auris virulence factors (Figure 2) [41].
In terms of stress sensitivities of C. auris, Hog1-related stress-activated protein kinase is an important virulence trait for fungal survival against host-imposed stresses and are highly required for the pathogenicity of many fungal pathogens during infection [42]. Day et al. showed that Hog1 is involved in regulating stress resistance, cell morphology, aggregation, and virulence in C. auris [43].
With regard to biofilm formation, in contrast to C. albicans which forms the heterogeneous architecture of biofilms combined with blastoconidia and hyphae embedded within the extracellular matrix, C. auris produces thin biofilms composed mostly of blastoconidia and occasionally pseudohyphae embedded within very limited extracellular matrix [40]. Interestingly, these C. auris biofilms display lower susceptibility against antifungals including polyenes, azoles, echinocandins and chlorhexidine when compared to those of C. albicans suggesting other mechanisms to be more important for this antifungal-resistant biofilm than the reduced biomass of C. auris or limited extracellular matrix [43,44,45]. Additionally, adhesion plays a key role in C. auris virulence and biofilm formation. Agglutinin-like sequence (ALS) proteins, in particular Als3, are involved in C. auris adherence [46]. Singh et al. showed that sera containing anti-Als3 antibodies prevent C. auris biofilm formation supporting an important role of Als3 in biofilm formation [47]. Knowing the specificity of C. auris and its virulence factors, different strategies are conducted to fight this emerging superbug.
3. Repurposing Drugs
3.1. Drugs: In Vitro Screening
Repurposing of drugs is an attractive pathway to discover new applications to already developed and filed drugs. Drug repositioning could help spare substantial costs linked to new drug discovery and development. It is also highly interesting because it involves the use of de-risked compounds, avoiding high attrition rate [47,48]. Therefore, this strategy has been applied for repurposing of drugs against C. auris. It is notable that the reported studies have been mainly conducted in vitro unless specified.
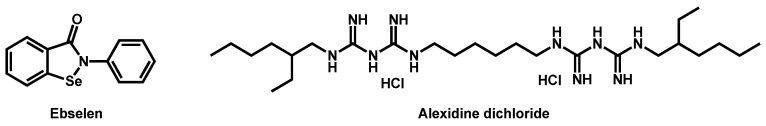
In that context, the Prestwick Chemical library®, a repurposing library of 1280 small mainly off-patent molecules, has been screened several times by different research groups. In 2018, Wall et al. identified, from this library, ebselen as a repositionable molecule [49]. Ebselen is a synthetic organoselenium drug molecule with antioxidant, anti-inflammatory, and cytoprotective activity (Figure 3). It exhibited 100% inhibition of growth of C. auris at a concentration of 2.5 µM. In addition, ebselen also inhibited the formation of biofilm and was defined as a broad-spectrum antifungal, not only active on Candida species, but also against a variety of medically important fungi, including yeasts and molds.
Figure 3.
Structures of ebselen and alexidine dichloride.
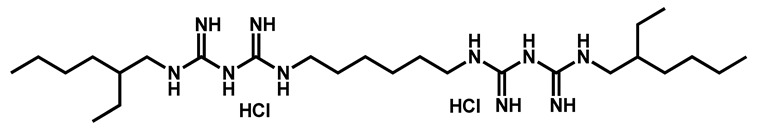
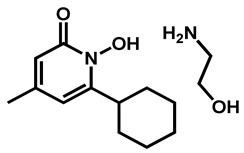
Mamouei et al. recently reported the results issued from their own screening of the same chemical collection against C. auris [50]. They focused on inhibitors able to block the fungal growth and to kill preformed biofilms. The bis-biguanide alexidine dihydrochloride (AXD) (Figure 3), a potent and selective PTPMT1 (Protein Tyrosine Phosphatase Localized to the Mitochondrion 1) inhibitor, exhibited the highest antifungal and antibiofilm activity against a panel of pathogens, including C. auris.
In parallel, from the Prestwick Library®, a major work dealing with repurposing of compounds to fight specifically against C. auris has been conducted by Zagaroza et al. in 2019 [51]. From this massive screening, 27 drugs proved to inhibit the growth of three different strains of C. auris with different geographical origin: CL-10093, JCM 15448, and KCTC 17810 (Table 1). From this pre-selection, the scientists carried complementary studies on 10 drugs, namely MK 801 hydrogen maleate, ciclopirox ethanolamine, trifluoperazine dihydrochloride, suloctidil, ebselen, tamoxifen citrate, rolipram, thiethylperazine dimalate, guanadrel sulfate, and pyrvinium pamoate, and also recently confirmed the described repositionable compounds such as alexidine and ebselen.
Table 1.
Active repositionable drugs against C. auris according to Zagaroza et al.
| Structure | Name | Class | Growth Inhibition (%) at 50 µM | ||
|---|---|---|---|---|---|
| CL 10093 |
JCM 15448 | KCTC 17810 | |||
 Alexidine dihydrochloride |
Antibacterial | 97 | 97 | 97 | |
|
Artemisinin | Antimalarial | 65 | 56 | 61 |
 Benzethonium chloride |
Antibacterial | 96 | 97 | 100 | |
 Chlorhexidine |
Antibacterial | 98 | 99 | 98 | |
|
Chloroxine | Antibacterial | 95 | 97 | 98 |
|
Ciclopirox ethanolamine | Antibacterial/Antifungal | 94 | 97 | 94 |
|
Clioquinol | Antiamebic/Antibacteria | 89 | 93 | 93 |
 Dequalinium dichloride |
Antibacterial | 81 | 86 | 89 | |
|
Dimethisoquin hydrochloride | Antipruritic | 59 | 65 | 51 |
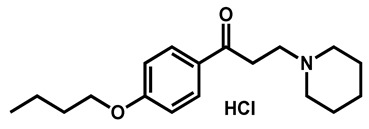
|
Dyclonine hydrochloride | Local anesthetic | 63 | 63 | 54 |
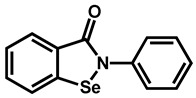
|
Ebselen | Anti-inflammatory | 87 | 91 | 92 |
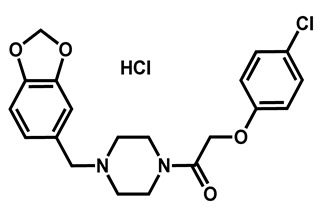
|
Fipexide hydrochloride | Anti-fatigue | 68 | 63 | 54 |
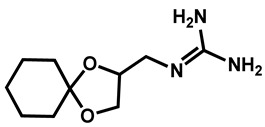
|
Guanadrel sulfate | Antihypertensive | 97 | 97 | 97 |
|
Hexachlorophene | Antiseptic | 97 | 98 | 86 |
|
Methyl benzethonium chloride | Antibacterial | 98 | 97 | 100 |
|
Methiothepin maleate | Antipsychotic | 74 | 72 | 64 |
|
MK 801 hydrogen maleate | Anticonvulsant | 98 | 98 | 97 |
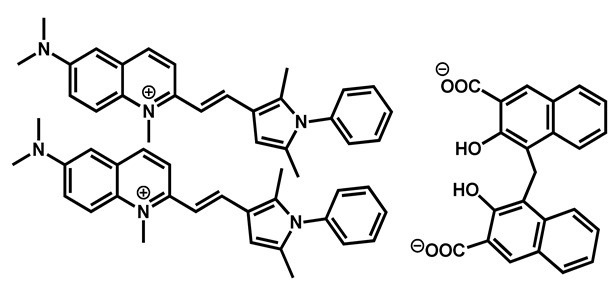 Pyrvinium pamoate |
Anthelmintic | 40 | 76 | 61 | |
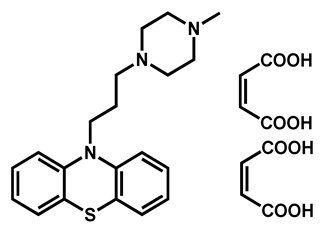
|
Prochlorperazine dimaleate | Antiemetic/Antipsychotic | 71 | 66 | 72 |
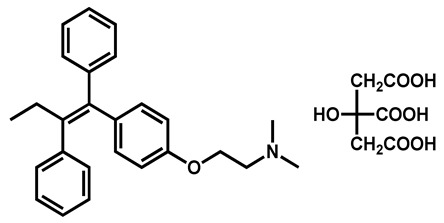
|
Tamoxifen citrate | Antineoplastic | 98 | 98 | 90 |
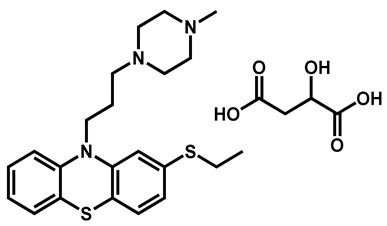
|
Thiethylperazine dimalate | Antiemetic | 68 | 90 | 86 |
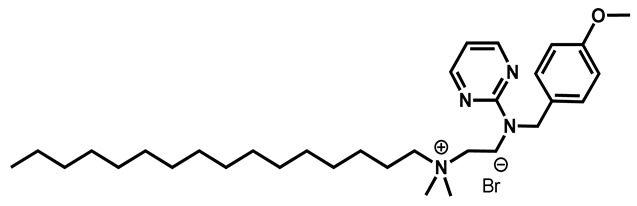 Thonzonium bromide |
Antiseptic | 98 | 98 | 98 | |
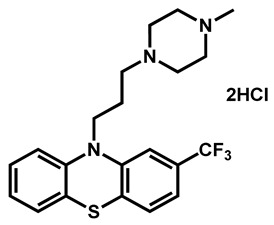
|
Trifluoperazine dihydrochloride | Antiemetic | 54 | 88 | 61 |
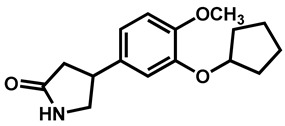
|
Rolipram | Antidepressant | 98 | 97 | 91 |
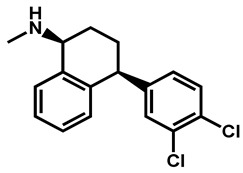
|
Sertraline | Antidepressant | 59 | 88 | 56 |
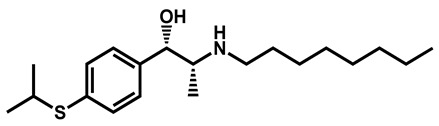
|
Suloctidil | Antiplatelet | 96 | 99 | 78 |
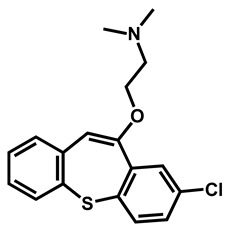
|
Zotepine | Antipsychotic | 59 | 63 | 56 |
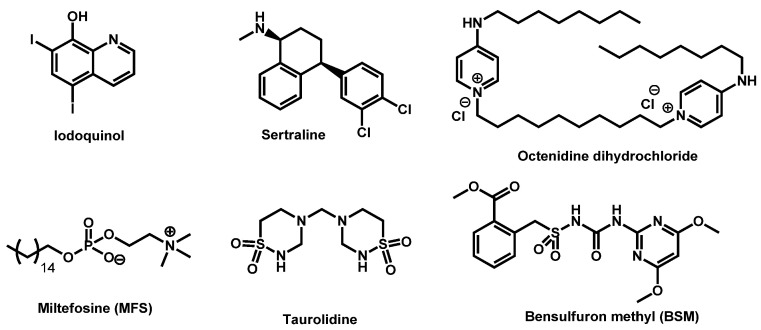
Gowri et al. studied sertraline, a repurposing drug classically used for the treatment of depression, against three different isolates of C. auris (Figure 4). Sertraline demonstrated to effectively kill C. auris but also to inhibit the formation of biofilm. Its mode of action was elucidated by in silico studies and revealed the binding nature of sertraline to the sterol 14 α-demethylase which is involved in ergosterol biosynthesis [52].
Figure 4.
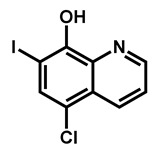
Structures of iodoquinol, miltefosine, sertraline, taurolidine, octenidine dihydrochloride and bensulfuron methyl.
Taurolidine and its derivatives have been recently patented by Diluccio and Reidenberg for the treatment of blood infection by C. auris [53]. Taurolidine (Figure 4) is an antimicrobial that is used to try to prevent infections in catheters.
Octenidine dihydrochloride is a cationic gemini-surfactant, derived from pyridine (Figure 4). More than two decades ago, it was designed for skin, mucous membrane and wound antisepsis. It is currently used as antiseptic in a large field of applications as alternative to chlorhexidine, polyvidone-iodine or triclosan [54]. In 2019, Ponnachan et al. reported the ability of octenidine dihydrochloride to kill C. auris strains [55].
Fifty commercially available herbicides, targeting acetohydroxyacid synthase, were recently evaluated by Guddat et al. against C. auris CBS10913 strain. Among these compounds, bensulfuron methyl (BSM), belonging to the sulfonylurea chemical subfamily was the most potent discovered antibiofilm formation and antifungal agent (Figure 4) [56].
3.2. Drugs: In Vitro Screening and In Vivo Validation
Using medicines for malaria venture’s pathogen box, Wall et al. recently confirmed iodoquinol and miltefosine as potent inhibitors of C. auris strain 0390, both under planktonic and biofilm growing conditions (Figure 4) [57]. Both compounds possess broad-spectrum of activity against Candida spp., including multiple strains of the emergent C. auris, irrespective of their resistance profiles. Miltefosine (MFS) was also reported in combination with amphotericin B [58]. In the last few months, Barreto et al. confirmed the interest of MFS as an alternative approach to fight against the emerging fungus C. auris [59]. They reported its fungicidal activity against planktonic cells of C. auris clinical isolates, and antibiofilm ability. They also studied the encapsulation of MFS in alginate nanoparticles (MFS-AN). Using a Galleria mellonella larvae infected by C. auris model, they demonstrated that both MFS and MFS-AN were able to increase the survival rate. The main advantage of MFS-AN over MFS was its reduced toxicity.
3.3. Vaccines: In Vivo Evaluation
Ibrahim et al. reported the efficacy of NDV-3A vaccine to protect mice from multidrug resistant Candida auris infection [43,60]. NDV-3A vaccine, harboring the N-terminus of Als3p formulated with alum, has been developed initially against C. albicans. The authors proved that it generated cross-reactive antibodies against C. auris clinical isolates and protected neutropenic mice from C. auris infection. Moreover, this repositioning vaccine displayed an additive protective effect in neutropenic mice when combined with micafungin. This vaccine alternative has been recently filed [46].
3.4. Critical Analysis of Repurposing Drugs from a Chemical/Physicochemical Point of View
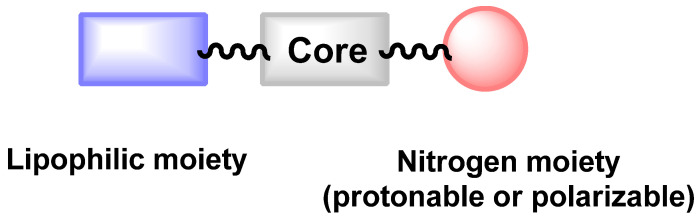
From a chemical point of view, it has to be mentioned that the chemical diversity of reported antifungal active against C. auris is quite low. Only 30 repositionable compounds have been described for their activity against C. auris and most of them can be gathered based on common chemical features: quinoline/isoquinoline, guanidine, phenothiazine/benzothiepine or amide-based cores. These cores are decorated with protonable/polarizable nitrogen groups (pyridine, ternary amine, ammonium, piperazine) and lipophilic moieties (fatty chain or halogenated aromatics). This could be summarized as followed in Figure 5. The protonable nitrogen could be part of the core moiety as in quinoline/isoquinoline derivatives.
Figure 5.
Proposed scaffold of reported C. auris antifungals.
The reported repositionable compounds have been gathered in Table 2, Table 3 and Table 4 and Figure 5 and Figure 6 with analysis of their composition according to the proposed scaffold.
Table 2.
Critical analysis of quinoline/isoquinoline repositionable drugs.
| Entry | Structures | Log P 1 | TPSA 2 (Å) |
M (g/mol) |
Druglikeliness 3 (Alert) |
BBB Permeant 4 | GI Absorption 5 |
|---|---|---|---|---|---|---|---|
| 1 |
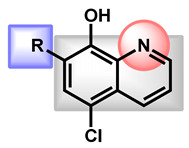 R = I = Clioquinol R = Cl = Chloroxine |
2.96 | 33.12 | 305.50 | XLOGP > 3.5 | Yes | High |
| 2 | 2.86 | 33.12 | 214.05 | MW < 250, XLOGP > 3.5 | Yes | High | |
| 3 |
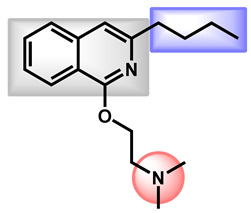 Dimethisoquin Dimethisoquin |
3.66 | 25.36 | 272.39 | XLOGP > 3.5 | Yes | High |
| 4 |
 Dequalinium dichloride Dequalinium dichloride |
4.14 | 59.80 | 456.67 | MW > 350 Rotors > 7 XLOGP > 3.5 |
No | High |
| 5 |
 Pyrvinium active compound Pyrvinium active compound |
4.29 | 12.05 | 382.52 | MW > 350 XLOGP > 3.5 |
Yes | High |
1. LogP = octanol/water partition coefficient calculated by SwissADME; 2 TPSA = topology polar surface area by SwissADME; 3 Druglikliness = criteria which should be problematic for drug development according to SwissADME; 4 BBB permeant: blood brain barrier permeant as predicted by SwissADME; 5 gastro-intestinal permeation ability as predicted by SwissADME.
Table 3.
Critical analysis of benzothiepine/phenothiazine repositionable drugs.
| Entry | Structure | R | LogP 1 | TPSA (Å) 2 | M (g/mol) |
Druglikeliness (Alert) 3 |
|---|---|---|---|---|---|---|
| 1 |
|
-S-CH3 (Methiothepin) |
3.94 | 57.08 | 356.55 | MW > 350, XLOGP > 3.5 |
| 2 | -Cl (Zotepine) |
4.37 | 37.77 | 331.86 | XLOGP > 3.5 | |
| 3 |
|
-H (Prochlorperazine) |
3.47 | 35.02 | 339.50 | XLOGP > 3.5 |
| 4 | -S-CH2CH3 (Thiethylperazine) |
4.43 | 60.32 | 399.62 | MW > 350, XLOGP > 3.5 | |
| 5 | -CF3 (Trifluoperazine) |
4.53 | 35.02 | 407.50 | MW > 350, XLOGP > 3.5 |
1. LogP = octanol/water partition coefficient calculated by SwissADME; 2 TPSA = topology polar surface area by SwissADME; 3 Druglikliness = criteria which should be problematic for drug development according to SwissADME.
Table 4.
Critical analysis of amide-based repositionable drugs.
| Entry | Structures | LogP 1 | TPSA 2 (Å) |
M (g/mol) | Druglikeliness 3 (Alert) |
BBB Permeant 4 | GI 5 Absorption |
|---|---|---|---|---|---|---|---|
| 1 |
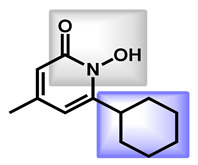 Ciclopirox |
2.38 | 42.23 | 207.27 | MW < 250 | Yes | High |
| 2 |
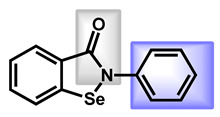 Ebselen |
1.75 | 22.0 | 274.18 | No alert | Yes | High |
| 3 |
 Rolipram Rolipram |
2.44 | 47.56 | 275.34 | No alert | Yes | High |
| 4 |
 Fipexide Fipexide |
2.82 | 51.24 | 388.84 | MW > 350 | Yes | High |
| 5 |
 Taurolidine |
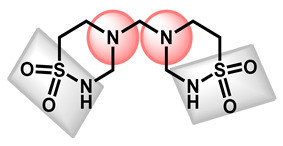
−1.53 | 115.58 | 284.36 | 0 alert | No | Low |
1. LogP = octanol/water partition coefficient calculated by SwissADME; 2 TPSA = topology polar surface area by SwissADME; 3 Druglikliness = criteria which should be problematic for drug development according to SwissADME; 4 BBB permeant: blood brain barrier permeant as predicted by SwissADME; 5 gastro-intestinal permeation ability as predicted by SwissADME.
Figure 6.
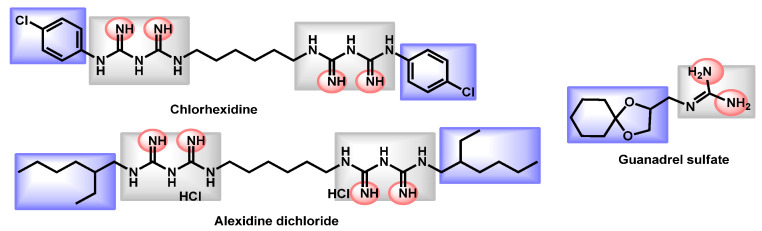
Critical analysis for the guanidine derivatives.
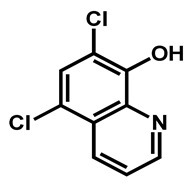
Concerning the quinoline/isoquinoline sub-group, composed of five molecules, it is notable than the simplest drugs—clioquinol and chloroxine—differed in only one halogen atom. Indeed, the iodine in clioquinol is replaced by a chlorine in chloroxine. This slightly impacted the lipophilicity of the compound as chloroxine is a little more hydrophilic than clioquinol. However, these two compounds could be referred as analogs (Table 2, Entries 1 and 2). In dimethisoquin, the quinoline core is substituted by an additional dimethylamine group which is easily protonable and a fatty butyl chain to enhance the lipophilicity (Table 2, entry 3). Dequalinium dichloride is a bis-quinoline derivative than perfectly fit the proposed scaffold (Table 2, entry 4). In the same manner, pyrvinium pamoate respects the defined features (Table 2, entry 5). On the whole, the quinoline/isoquinoline series is composed of lipophilic compounds (2.86 < logP < 4.29).
Concerning the guanidine derivatives, the same features are also represented (Figure 6). The guanidine core serves also as the protonable part. From a descriptor point of view, these compounds are not blood brain barrier (BBB) permeants. The bis-biguanidines (chlorhexidine and alexidine) are quite lipophilic with logP equal to 2.79 and 4.67, respectively. However, guanadrel is quite balanced (logP = 0.59). The three drugs exhibited high TPSA (82.86 to 167.58 Å).
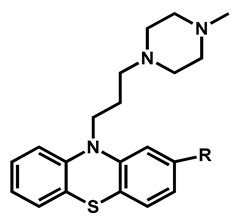
Heterocyclic sulfur compounds bearing a phenothiazine or a benzothiepine core displayed good activities against C. auris. All are highly absorbed in the gastrointestinal tract and can pass the BBB. The five reported compounds could be divided into two sub-classes and differed only by the nature of the R-substitution on the aromatic moiety (Table 3). Two benzothiepine derivatives (methiothepin and zotepine) were reported. Both are lipophilic (logP > 3.9) and exhibited a protonable N-methylpiperazine ring as the side-chain. The same N-methylpiperazine moiety is kept in the phenothiazine derivatives (prochlorperazine, thiethylperazine, trifluoperazine) with logP ranging from 3.47 to 4.53. The introduction of more lipophilic substituents—ethylsulfur and trifluoromethyl—increased the activity against C. auris JCM 15448 strain particularly.
The last sub-group which could be addressed is based on an amide fragment. The following repositionable drugs have been also analyzed (Table 4). In that case, the polar amide bond could replace the nitrogen-protonable part. However, lipophilic moieties are clearly present, such as para-chlorobenzyl or cyclohexyl groups. It is notable that taurolidine, a bis-sulfonamide drug, is the only repositionable drug, which is hydrophilic, with low GI absorption.
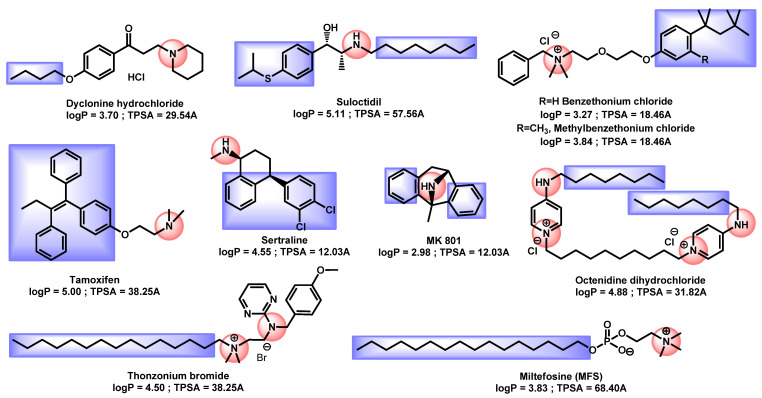
All others repositionable compounds except artemisin and hexaclophene exhibited a nitrogen protonable group or a quaternary ammonium coupled with a lipophilic moiety even if no common core can be distinguished (Figure 7). Their logP ranged from 2.98 to 5.11, proving their lipophilicity.
Figure 7.
Critical analysis for repurposing drugs.
4. Combination Drugs
Because discovering co-drugs capable of overcoming resistance to frontline antifungals is of prime clinical importance, combination drugs were often reported during the last five years. The potential combinations could be between known antifungal agents (Section 4.1), between repurposing drug and one antifungal (Section 4.2) or between a novel active compound and an “old” antifungal (Section 4.3).
4.1. Combination of Antifungal Drugs
To date, only in vitro results are described in this part.
In 2017, Fakhim et al. reported the effects of combination between echinocandins and triazoles against 10 multidrug-resistant C. auris isolates [61]. Using a microdilution checkerboard technique, they screened the in vitro interactions of azoles and echinocandins in indian clinical isolates resistant to fluconazole and/or micafungin. They discovered promising synergistic interactions for two couples: micafungin/voriconazole and micafungin/fluconazole. This strategy has also been demonstrated recently by the in vitro synergistic combination of isavuconazole (azole derivative) with colistin [62].
In 2020, O’Brien et al. tested if two-drug combinations were effective in vitro against multidrug-resistant C. auris isolates [63,64]. From nine reference antifungals and around a thousand tested combinations, they reported that flucytosine (5FC) at 1.0 µg/mL potentiated the most combinations, especially in the case of amphotericin B, echinocandins and voriconazole resistant strains where its addition allowed to restore the fungicidal activity.
4.2. Combinations of a Repositioning Drug with an Antifungal Drug
4.2.1. In Vitro Screening
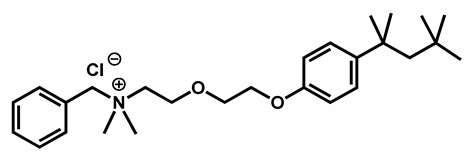
In 2018, Seleem et al. studied the combinations between sulfa drugs and reference azole antifungals. Among the active sulfa drugs, the bacteriostatic antibiotic sulfamethoxazole exhibited the most potent in vitro synergistic interactions with voriconazole and itraconazole (Figure 8). The addition of sulfamethoxazole restored the activity of azole drugs against azole-resistant strains if the resistance originated from either overproduction of or decreased affinity for the azole target (ERG11p). Strains resistant because of efflux pump hyperactivity were not susceptible to these combinations [65].
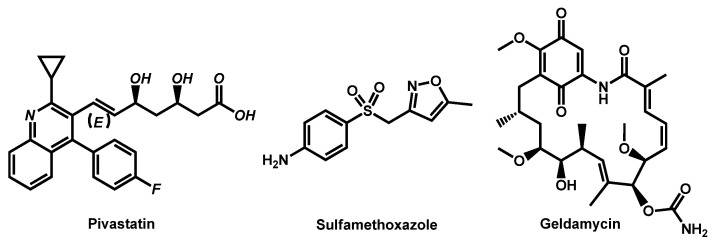
Figure 8.
Structure of Pivastatin and Sulfamethoxazole.
In a parallel manner, in 2020, the same team from Seleem, Mayhoub et al. evaluated the ability of Pharmakon® 1600 drug library to sensitize an azole-resistant C. albicans to the effect of fluconazole [66]. They discovered that pitavastatin, a relatively newly developed cholesterol lowering agent, was a potent azole chemosensitizing agent (Figure 8). From a chemical point of view, pivastatin belongs to the quinoline sub-group, including some repurposing drugs. Pivastatin displayed broad-spectrum synergistic interactions with both fluconazole even against C. auris strains (MIC from 8 to 64 µg/mL for combination against 64 to 256 µg/mL for drugs alone). Moreover, pitavastatin-fluconazole combination significantly reduced the biofilm-forming abilities of the tested Candida species by up to 73%.
In 2019, the team of Chowdhary reported the in vitro effects of combination between geldanamycin (Hsp90-inhibitor) with triazoles and echinocandins against common and emerging Candida species (Figure 8) [67]. Whereas synergistic interactions between geldamycin and antifungal drugs were observed for C. albicans, C. glabrata and C. parapsilosis, no interesting effect was observed for any combination against C. auris.
Dannaoui et al. evaluated the in vitro interaction between colistin and two echinocandins (caspofungin and micafungin) against 15 C. auris isolates [68]. Colistin, or polymyxin E, is a last-resort antibiotic against multidrug resistant gram-negative infections (Figure 9). It displayed no activity with MIC of >64 μg/mL for all the isolates. However, when colistin was combined with caspofungin, synergistic interactions were observed for all strains.
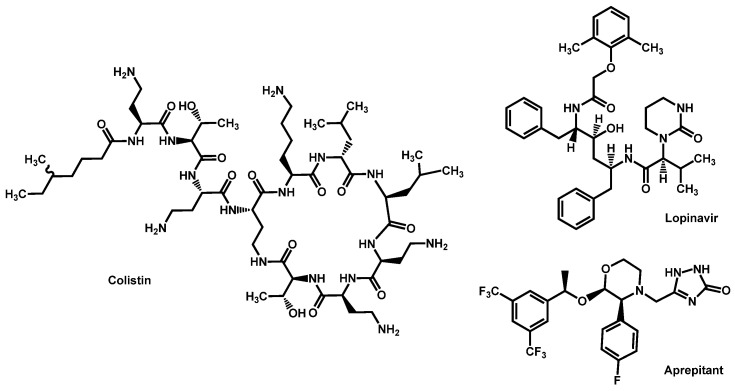
Figure 9.
Structure of Colistin, Lopinavir and Aprepitant.
Very recently, Seleem et al. demonstrated that aprepitant, an antiemetic agent, is a novel broad-spectrum azole chemosensitizing agent (Figure 9). The combination aprepitant/itraconazole is particularly efficient in an in vivo C. elegans model of infection by C. auris. This synergistic relationship could be mediated by interferences with metal ion homoestasis and subsequent impact on ROS production [69]. The same team also reported that lopinavir, an HIV protease inhibitor, is able to chemosensitize azole drug.
4.2.2. In Vivo Confirmation
Following the report by Seleem et al., the synergy between sulfamethoxazole and voriconazole was confirmed in vivo in a C. elegans model of C. auris infection [65].
Moreover, the same group demonstrated in vivo that the lopinavir/itraconazole combination decreased the fungal burden in C. auris-infected C. elegans nematodes by 88.5% and enhanced their survival rate by 90% relative to that of the untreated control [70]. Comparing lopinavir and aprepitant, both studies reported by the same team on the same in vivo protocols, lopinavir seems to be more potent than aprepitant in the optimized combination with itraconazole.
4.3. Combination of New Compounds and Old Drug: In Vitro Results
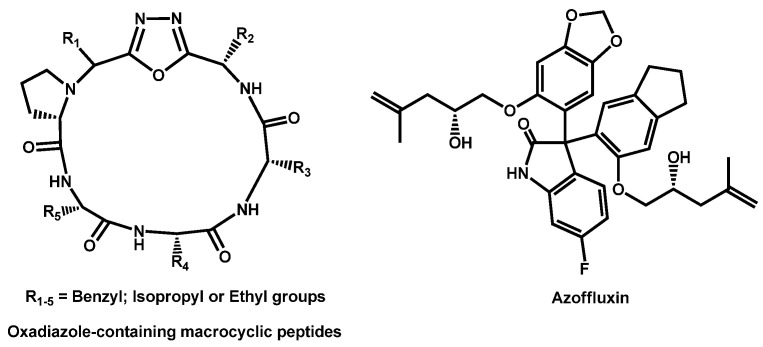
Revie, Robbins et al. recently described the ability of oxadiazolecontaining macrocyclic peptides to potentiate the antifungal activity of fluconazole [71]. Few of the newly obtained oxadiazole-containing macrocyclic peptides displayed activity against Candida spp on their own, but many increased the efficacy of fluconazole, resulting in a synergistic combination (Figure 10).
Figure 10.
Oxadiazole-containing macrocylic peptides and azoffluxin.
The synthetic library created by Boston University’s Center for Molecular Discovery® (BU-CMD) was screened at 50 µM to identify azole-synergizing compound. Azoffluxin, due to its strong synergistic interaction with fluconazole against a resistant strain of C. auris Ci6684 was identified (Figure 10). Azoffluxin enhances azole efficacy in a Cdr1-dependent manner [72].
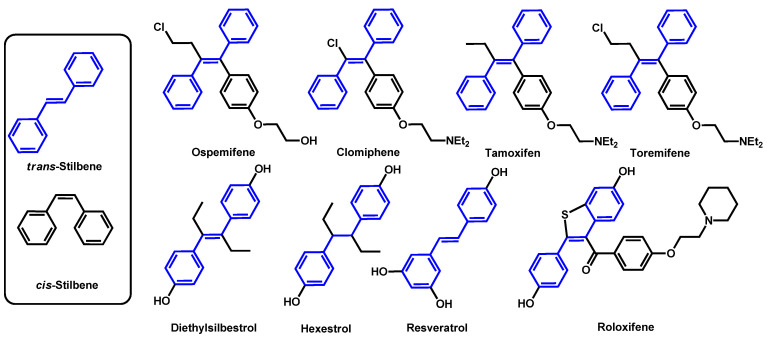
Seleem et al. tested nine stilbene compounds for their ability to interact synergistically with azole drugs, particularly against azole-resistant fungal isolates (Figure 11) [73]. Ospemifene displayed the most potent azole chemosensitizing activity and increased the potency of itraconazole against C. auris. Indeed, when MIC are superior to 256 µg/mL for both ospemifene and itraconazole alone, their combination exhibited a MIC equal to 2 µg/mL. Moreover, the authors determined that ospemifene interfered directly with fungal efflux systems, thus allowing the fungal intake of itraconazole.
Figure 11.
Stilbene compounds tested by Seelem et al. in combination with itraconazole.
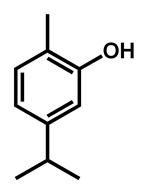
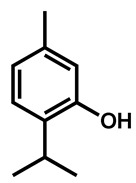
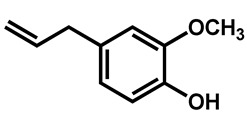
Ahmad et al. examined the effects of combination of natural monoterpene phenols with antifungal drugs against 25 clinical isolates of C. auris [74]. None of the monoterpene phenols was active alone against C. auris (Table 5). Carvacrol was the most active phenol with median MIC of 125 µg/mL and its combination with amphotericin B, nystatin, fluconazole and caspofungin resulted synergistic and additive effects in 64%, 96%, 68%, and 28%, respectively.
Table 5.
Antifungal activity of phenolic compounds against C. auris as reported by Ahmad et al.
| Test Agents | Structure | MIC Values (µg/mL) (n = 3) | |
|---|---|---|---|
| Median (Range) | |||
| C. auris | Carvacrol |
|
125 (62–250) |
| Thymol |
|
312 (156–625) | |
| Eugenol |
|
625 (312–1250) | |
| Methyl eugenol |
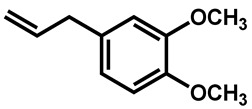
|
1250 (625–1250) |
5. Novel Compounds
In this section, we reviewed series of novel compounds which have displayed a good to potent activity against C. auris. For each chemical class, a critical analysis based on its chemicophysical descriptors will be performed for comparison purpose. The first section is devoted to compounds with potent in vitro activities, whereas the second section concerns chemical classes with proved in vivo efficacy.
5.1. Small Molecules
5.1.1. In Vitro Evaluation
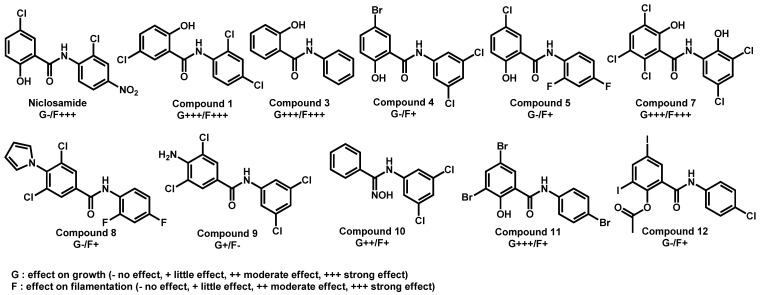
In 2018, in order to target virulence traits, Selam et al. described the screening of a 698 small-molecule collection against the invasive hyphal growth of C. albicans [75]. From their data, niclosamide, an FDA-approved anthelmintic in humans and one of its analogue (N1-(3,5-dichlorophenyl)-5-chloro-2-hydroxybenzamide), an halogenated salicylanilide (1)—emerged as capable of inhibit both filamentation and biofilm formation. Based on these preliminary results, they extended their work to a series of a dozen of compounds to improve their biological activities. Therefore, they brought to light compounds 3 and 7, which displayed both anti-filamentation and biofilm formation capacities (Figure 12). This series of compounds are amide-based small molecules with balanced lipophilicity induced by addition of halogenated substituents. They could be related to the previously described amide-based repurposing drugs. As example, logP of Compound 1 was calculated at 4.10 and its topological polar surface area equal to 49.33Å, which is in accordance with the values observed for repurposing drugs active against C. auris.
Figure 12.
Series of derivatives from niclosamide and their activities towards growth and filamentation as reported by Selam et al.
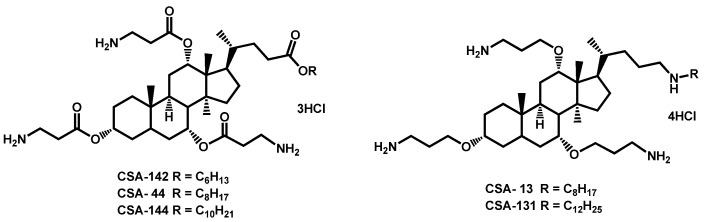
In 2018, Genberg et al. also filed a patent concerning methods for treating fungal infections using a cationic steroid antimicrobial (CSA) [76]. Some cationic steroid antimicrobials have been described and exhibited a broad-spectrum of activity against a panel of fungi, including Candida spp. (Figure 13). In such structures, the steroid core is highly lipophilic and the aminoalkyloxychains could bring a little hydrophilicity and also protonable primary or secondary amino groups.
Figure 13.
CSA steroids as patented by Genberg, Beus and Savage and MYC-053 as reported by Tetz.
In 2019, Tetz et al. described the in vitro activity of a novel compound named MYC-053 (Figure 13) against clinically significant multidrug resistant strains such as C. glabrata, C. auris, C. neoformans and Pneumocystis spp. [77,78]. Under their screening conditions, MYC-053 proved to be equally effective against both susceptible control strains, clinical isolates, or resistant strains and exhibited MICs ranging from 0.125 to 4.0 µg/mL. The profile of activity of MYC-053 is unique, because, unlike other antifungals such as azoles, polyenes, and echinocandins, MYC-053 was effective against Pneumocystis isolates. The compound is currently under development by TGV-Therapeutics. MYC-053, as a sodium salt, is hydrophilic (logP = −0.60). Its topological polar surface area is equal to 78.76 Å. The pyrimidine dione core (in grey) is quite similar to amide-based derivatives and the 2-hydroxy-3,5-dichlorophenyl moiety helped to increase the lipophilicity.
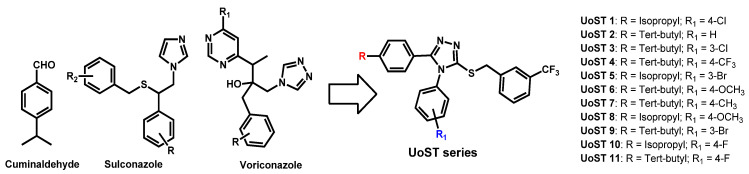
In 2020, Soliman et al. described the chemical association of the essential oil cuminaldehyde and azole moieties (as present in sulconazole or voriconazole) in order to take advantage of their individual previously reported anti-candidal properties (Figure 14) [79]. UoST series was evaluated against C. albicans and C. auris. Only UoST5, UoST7, UoST8 and UoST11 displayed good activity against C. auris (MIC ranging from 2 to 15 µg/mL). Obtained by a six-steps pathway, UoST5, their best designed hybrid molecule exhibited a 2 µg/mL MIC50 against C. auris where amphotericin B displayed MIC50 = 0.3 µg/mL. Compound UoST5 was then formulated into PLGA nanoparticles (NPs) and this formulation proved to enhance and prolong the anti-candidal activities of UoST5. OsST5 is a very lipopholic triazole-based compound (logP = 7.11) with moderate polar surface area (TPSA = 56.01).
Figure 14.
Rational design of cuminaldehyde-azole hybrid compounds by Soliman et al.
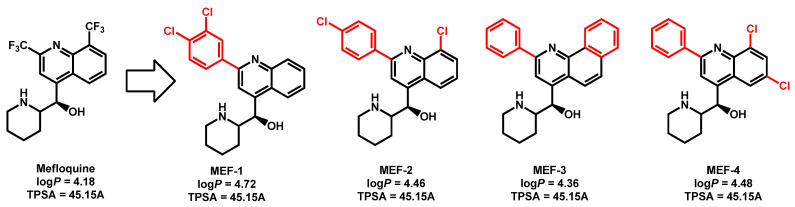
Montoya et al. reported that derivatives of the anti-malarial drug mefloquine have broad-spectrum antifungal activity against pathogenic yeasts and molds [80]. These derivatives were tested against a panel of fungal strains, including C. auris strain 0381 and they revealed MIC from 2–4 µg/mL compared to MIC = 128 µg/mL for mefloquine itself (Figure 15). From a chemical point of view, mefloquine and its derivatives belongs to the quinoline chemical class. They displayed the three features defined previously: (i) quinoline core; (ii) lipophilic moiety (halogenated aromatics) and (iii) piperidine protonable nitrogen group. Mefloquine derivatives are quite lipophilic (logP from 4.36 to 4.72) with moderate TPSA.
Figure 15.
Structure of the derivatives of mefloquine as reported by Montoya et al.
Orofino et al. reported in 2020 the potency of BM1, a macrocyclic amidinourea active against azole-resistant Candida strains [81]. The authors conducted a complete characterization of the in vitro and in vivo biological properties of BM1 and established its good ADME and biochemical characteristics so as its high activity towards several Candida species, including preliminary data against C. auris (Table 6). BM1 exhibited a logP of 3.38 and a very high topological polar surface area of 116.13 Å. From a chemical point of view, it belongs to the guanidine derivatives.
Table 6.
Antifungal activities of compound BM1 against various Candida species.
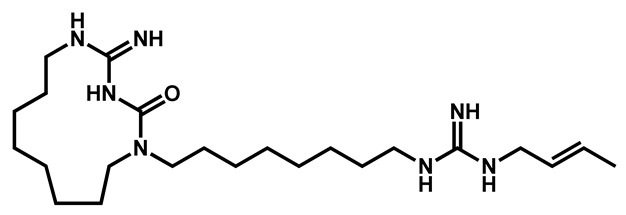 BM1 BM1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC and MBC Rage (µg/mL) against Candida Species | |||||||||||
| C. albicans | C. tropicalis | C. parapsilosis | C. glabrata | C. krusei | C. auris | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| 0.125–2 | 2–4 | 2 | 2–4 | 8–16 | 32–64 | 32–64 | 8–64 | 8–64 | 64–128 | 8–64 | 128–256 |
MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
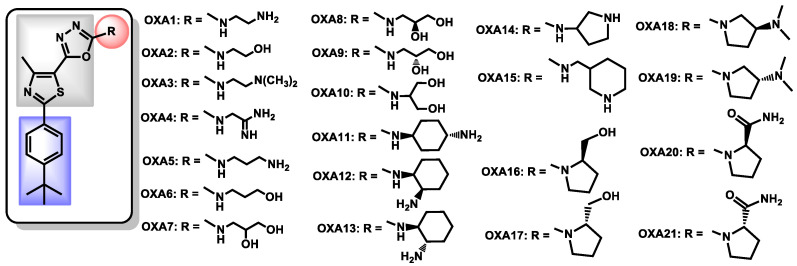
Seleem, Mayhoub et al. are widely involved in the development of polysubstituted thiazoles as novel antibacterial agents. Based on structure-activity relationships studies, they recently described oxadiazolylthiazoles as novel and selective antifungal agents [82]. Via a five-steps pathway, the authors obtained a series of oxadiazolylthiazole OXA1-21 (Figure 16) and evaluated them against a panel of fungal and bacterial strains. OXA11 proved to be selective towards fungal strains and the most active compound from this series, with MIC = 2–4 µg/mL against C. auris strains. Moreover, OXA11 exhibited a fungistatic behavior, a broad-spectrum activity against fungal pathogens, with capability of disrupting biofilms but without harming the human normal microbiota. The OXA derivatives perfectly respect the three common features: (i) an original oxadiazolylthiazole core (in grey) coupled with (ii) a lipophilic moiety (in blue) and (iii) a protonable amine group (cyclic or acyclic). OXA11, the most potent reported molecule from this series, displayed logP equal to 4.44, a high topological surface area of 118.10 Å and no alert concerning its drug likeliness.
Figure 16.
Structures of OXA compounds described by Seleem, Mayhoub et al.
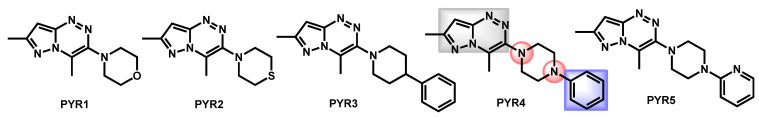
Seleem et al. also described the synthesis and biological evaluation of a small series of new pyrazolo[5,1-c][1,2,4]triazines against a panel of fungal strains, including C. auris (Figure 17) [83]. Compound PYR4 was the most potent antifungal, with a moderate MIC around 15–20 µg/mL. The chemical feature of PYR4 is composed of a pyrazolotriazine core, a piperazine protonable nitrogen group and a lipophilic aromatic (see Figure 17). PYR4, the most potent reported molecule from this series, displayed logP equal to 2.16, a medium topological surface area of 49.56 Å and no alert concerning its drug likeliness. However, compared to other series developed by Seleem et al., PYR derivatives are less powerful than OXA or PHE series.
Figure 17.
Series of pyrazolo[5,1-c][1,2,4]triazines described by Seleem et al.
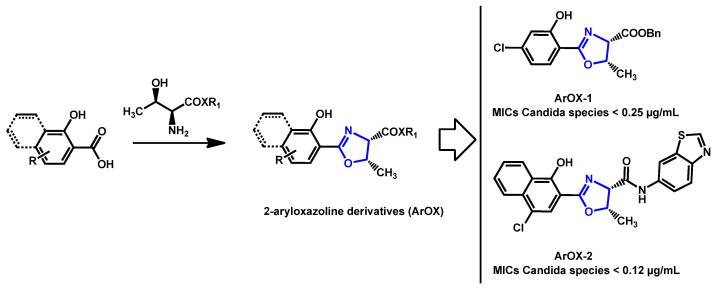
Late 2020, Ishida and Stephani reported 2-aryloxazoline compounds, obtained from L-threonine and aromatic carboxylic acid as promising antifungal compounds (Scheme 1) [84]. They discovered ArOX-1 and ArOX-2, which exhibited a strong in vitro antifungal activity, with MIC lower than 0.25 µg/mL for all tested Candida species. Moreover, these two non-toxic compounds were particularly active against C. auris CBS10913 (MICs = 0.06 µg/mL) and CBS12766 (MICs = 2 µg/mL).
Scheme 1.
Synthesis of 2-aryloxazoline derivatives (ArOx) and the two most potent compounds ArOX-1 and ArOx-2.
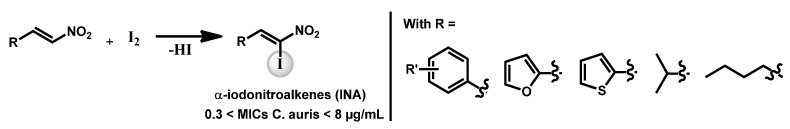
Ganesh et al. discovered a series of α-iodonitroalkenes as potential antifungal and antitubercular compounds. Based on a α-iodation on β-nitroalkenes, the authors obtained a series of derivatives (Scheme 2) which exhibited interesting antifungal activity against C. auris with MIC lower than 8 µg/mL for all compounds. It is notable than the furan and thiophene derivatives were the most broad-spectrum antifungals devoted of cytotoxicity [85].
Scheme 2.
α-Iodonitroalkenes synthesis.
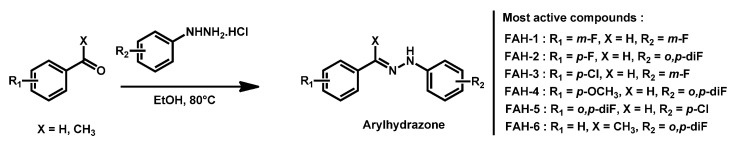
Among 40 tested hydrazone derivatives, fluorinated aryl- and heteroaryl-substituted hydrazone (FAH) have demonstrated broad-sprectrum fungicidal activities, antibiofilm capacity coupled with low cytotoxicity and did not trigger the development of resistance when exposed to C. auris (Scheme 3) [86]. Compounds FAH-1 to FAH-6 exhibited MIC < 1 µg/mL against nine of the 10 different C. auris strains. From a chemical point of view, these compounds fit the previously described model for C. auris antifungals.
Scheme 3.
Series of fluorinated hydrazone derivatives (FAH) as reported by Watt and Garneau-Tsodikova.
5.1.2. In Vitro Screening and In Vivo Validation
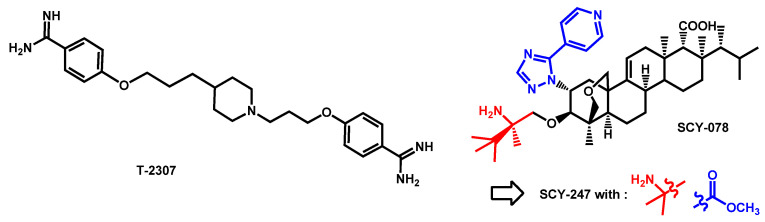
First described in 2008 by Mitsuyama et al. [87], T-2307, an arylamidine, exhibited potent broad-spectrum activities against the majority of fungal pathogens [88], even against echinocandin-resistant C. albicans and C. glabrata strains [89,90]. T-2307 is transported into C. albicans by a high-affinity spermine and spermidine carrier regulated by Agp2 [91], and causes collapse of mitochondrial membrane potential in yeast [92]. In 2020, Patterson et al. reported that arylamidine T-2307 as very active in vitro and in vivo antifungal against C. auris (Figure 12) [93]. In vitro MIC ranged from 0.125 to 4 μg/mL at 100% inhibition. Moreover, 3 mg/kg subcutaneous once daily treatment with T-2307 revealed an improved survival rate and reduced kidney fungal burden compared to control. In 2019, T-2307 was acquired by Appili Therapeutics Inc. for development and is now called ATI-2307.
From a chemical point of view, T-2307 displayed the features defined in the previous Figure 18. It exhibited two benzimidamide groups which are linked by a lipophilic propyl chain to a protonable N-subtituted piperidine. From this structure, compound T-2307 is rather like the guanidine sub-group.
Figure 18.
Structure of T-2307 and SCY-078 as reported by Patterson et al. and Larkin et al., respectively. Structure evolution of second generation fungerp SCY-247.
In 2017, Larkin et al. reported the effects of the orally available SCY-078, a novel 1,3-β-D-glucan synthesis inhibitor, on growth morphology and biofilm formation in C. auris (Figure 18) [40]. SCY-078 is a triterpene compound which differs from echinocandins by its oral bioavailability and its non-sensitivity towards the most common mutations within the protein target Fks. SCY-078 exhibited an MIC90 of 1 mg/liter against C. auris by interrupting cell-division. Additionally, SCY-078 displayed powerful antibiofilm activity, by reducing metabolic activity and thickness compared to the untreated control. In parallel, Berkow et al. demonstrated the in vitro activity of SCY-078 on a collection of 100 isolates, representative of the four clades of C. auris. The overall mode of SCY-078 was 1 µg/mL with MIC50 and MIC90 equal to 0.5 µg/mL and 1 µg/mL, respectively. SCY-078 also showed very little variation in activity between the clades compared to micafungin, caspofungin or anidulafungin [94]. Due to its first encouraging data, SCY-078 was referred as a promising new compound by McCarthy and Walsh in their review about new strategies and challenges against emerging and resistant fungal pathogens [95]. Since its discovery, SCY-078 (named Ibrexafungerp) has been integrated in a dozen of clinical trials studies and patented [96]. Concerning C. auris, one particular multicenter, open-label, non-comparator, single-arm study to evaluate the efficacy, safety, tolerability and pharmacokinetics of oral SCY-078 as an emergency use treatment for patients with a documented C. auris infection is still ongoing (ClinicalTrials.gov Identifier: NCT03363841). Recently, in 2020, Arendrup et al. investigated the in vitro activity of SCY-078 against C. auris by applying EUCAST methodology and compared its potential against C. albicans and C. glabrata and in reference with six control drugs (anidulafungin, micafungin, amphotericin B, fluconazole, voriconazole, and isavuconazole) [97]. More than 150 strains with various resistance profile were screened and proved to be uniformly susceptible to SCY-078. In parallel, Chaturvedi et al. reported that Ibrexafungerp was efficient against pan-resistant (defined as in vitro resistance to two or more azoles, all echinocandins and amphotericin B) C. auris isolates from the outbreak in New-York [98] and Ghannoum et al. demonstrated that it was active to control skin infection and colonization of hospitalized patients [99]. Recently, a review compiled data on Ibrexafungerp, established it is a promising, new antifungal agent to treat C. auris infections, as patients experienced a complete response after treatment [100].
From a chemical point of view, SCY-078 is based on a lipophilic tetracyclic-fused core with addition of protonable nitrogen moieties (pyridine, triazole and primary amine). Its structure is in accordance with the common defined scaffold.
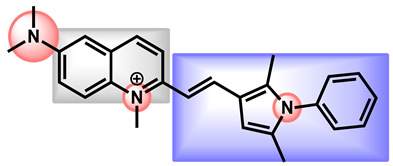
SCY-247, a second-generation fungerp which differs from SCY-078 by two chemical moieties (the 4-(1H-1,2,4-triazol-5-yl)pyridine and 2,3,3-trimethylbutan-2-amine moieties in SCY-078 that have been replaced by a methylester and a 2-methylpropan-2-amine in SCY-247, respectively (Figure 18) has been recently reported by Ghannoum et al. and proved similar activities against a panel of Candida strains and enhanced potency against C. auris strains. Moreover, SCY-247 retained its potential even when pH decreased [101].
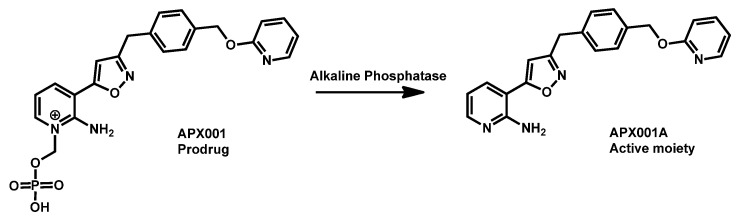
First reported in 2018, APX001A (formely E1210, Amplyx) is an antifungal agent that inhibits the fungal enzyme Gwt1 in the glycosylphosphatidylinositol (GPI) biosynthesis pathway. APX001 is the prodrug of APX001A (Scheme 4). Both APX001A and APX001 have been shown to be effective against a variety of fungal species, including Candida spp., Aspergillus spp. and other filamentous fungi. Compound APX001 has emerged as a novel potent antifungal agent against C. auris. It has been successfully tested against 100 geographically distinct clinical isolates and exhibited high MIC50 and MIC90 in the nanogram per liter range [102,103]. Extended evaluation of large panels of strains from different origins has confirmed the interest of APX001 in the treatment of infection by C. auris [104]. The compound APX001 has entered clinical trials and proved good efficacy in mice model of candidiasis [105,106]. Fosmanogepix (APX001) is currently in Phase II trials for invasive fungal infections. Both fosmanogepix and manogepix (APX001A) are currently under investigation and proofs of their in vivo efficacy against C. auris infection accumulate [105,106,107,108,109]. When analyzing its chemical structure, it appeared that APX001A displayed protonable nitrogen moieties (primary amine and pyridine) coupled with a lipophilic benzyl group. This balance gave a logP equal to 3.21 for compound APX001A with TPSA of 87.06 Å which is in the range observed for repurposing drugs. Its prodrug is much more polar (TPSA of 176.84 Å) and hydrophilic (logP = 1.70).
Scheme 4.
Structure of prodrug APX001 and active moiety APX001A.
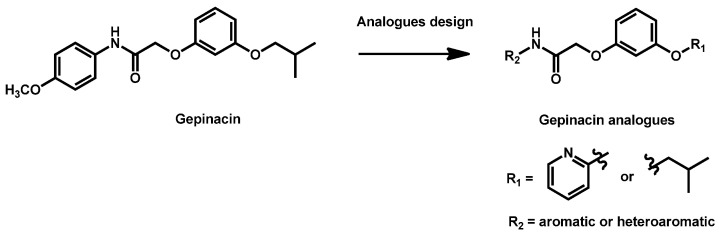
Gepinacin is a pre-clinical Gwt-1 inhibitor which is structurally different from manogepix. Despite high in vitro antifungal activity, gepinacin failed in in vivo trials due to its low metabolic and serum stability. A series of analogues was then designed and evaluated by Walsh and Cowen in 2020 (Scheme 5) [110]. A dozen of compounds have been obtained and exhibited MIC in the range 0.625–10 µg/mL against C. auris. However, despite interesting potency and promising single-dose pharmacokinetics, the identified lead (R1 = isobutyl; R2 = 4-chlorophenyl) did not reveal any activity at the maximal dose in a neutropenic rabbit model of disseminated candidiasis.
Scheme 5.
Structure of gepinacin and modulations.
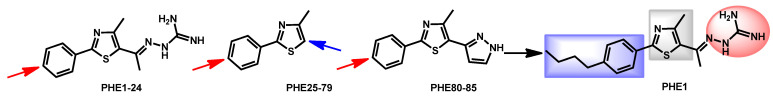
In 2019, the group headed by Seleem discovered that phenylthiazole small molecules possess dual antifungal and anti-biofilm activities against C. albicans and C. auris [111]. They tested 85 synthetic phenylthiazole derivatives (Figure 19) and compound PHE1 emerged as the most potent molecule, with MIC ranging from 0.25 to 2 µg/mL against a panel of C. albicans and C. auris strains. The HIT compound also exhibited anti-biofilm ability, fungicidal activity and ability to prolong survival in infected Caenorhabditis elegans model. From a chemical point of view, PHE1 perfectly fit the previously defined scaffold with a hydrazinecarboximidamide moiety (in red) which is a guanidine isostere, a thiazole core (in grey) and a 4-butylphenyl lipophilic chain (in blue). PHE1 displayed a logP = 3.64 and TPSA = 115.39 Å which are in accordance with the other reported drugs.
Figure 19.
Modifications around the phenylthiazole compounds as reported by Seleem et al.
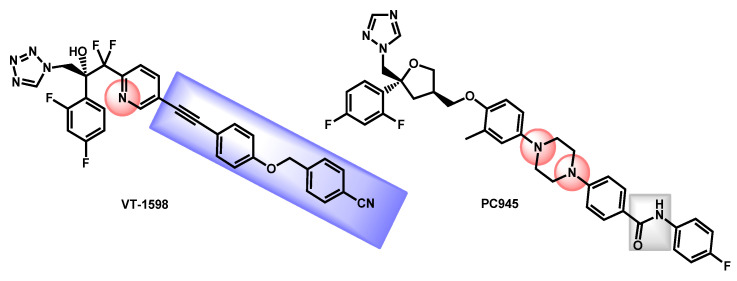
The work by Wiederhold et al. described the evaluation of VT-1598, a fungal CYP51-specific inhibitor against C. auris infections [112]. The tetrazole VT-1598, developed by Viamet Pharmaceuticals (Figure 20) has demonstrated in vitro activity against various fungi [113,114] and has also shown promising results in experimental models of invasive fungal infections such as central nervous system coccidioidomycosis or cryptococcal meningitis [115,116]. The authors demonstrated the in vitro and in vivo efficacy of VT-1598 against C. auris infections. VT-1598 is currently on Phase I clinical trials for coccidioidomycosis in order to evaluate its safety and PK of single oral doses in healthy patients (ClinicalTrials.gov Identifier: NCT04208321). From a chemical point of view, VT-1598 is a tetrazole-based compound which exhibited a protonable pyridine link and a lipophilic aromatic chain (in blue). Its lipophilicity (logP = 5.12) and polar surface (TPSA = 109.74 Å) are quite high and its gastrointestinal absorption is estimated as low.
Figure 20.
Structures of VT-1598 and PC945.
Still in 2019, Rudramurthy et al. described the activity of a novel topical triazole named PC945 against C. auris (Figure 20) [117]. PC945, optimized for topical application, proved to be 7.4-fold and 1.5-fold more potent than voriconazole and posaconazole against a collection of more than 50 clinical isolates from India, UK, USA, Japan and South-Korea. Three isolates were found to be cross-resistant to PC945 and other azoles but with no clear evidence of involved mutations. The tolerability, efficacy, and safety of PC945 were evaluated by four clinical trials in the frame of aspergillosis and candidiasis recently (ClinicalTrials.gov Identifier: NCT03870841; NCT03905447; NCT03745196 and NCT02715570). Three studies have been interrupted because of the COVID19 outbreak and results are not available. From a chemical point of view, PC945 exhibited a triazole ring—present in several antifungal references’ compounds—and an amide link. The integration of a piperazine link resulted in easily protonable nitrogen groups and fluorinated aromatics increased the lipophilicity. Its lipophilicity (logP = 5.70) and polar surface (TPSA = 84.75 Å) are quite high and its gastrointestinal absorption is estimated as low. These values and the presence of a 2,4-difluorophenyl moiety gave PC945 some similarities with VT-1598.
Overall, a dozen novel antifungal chemical families have been reported during the last few years and some of the lead products are currently under clinical trials investigations. From a chemical point of view, these compounds exhibited the same features as discovered repurposing compounds, divided into three complementary parts: (i) core; (ii) protonable nitrogen groups; (iii) lipophilic moieties (generally halogenated aromatics or fatty chains).
5.2. Echinocandins
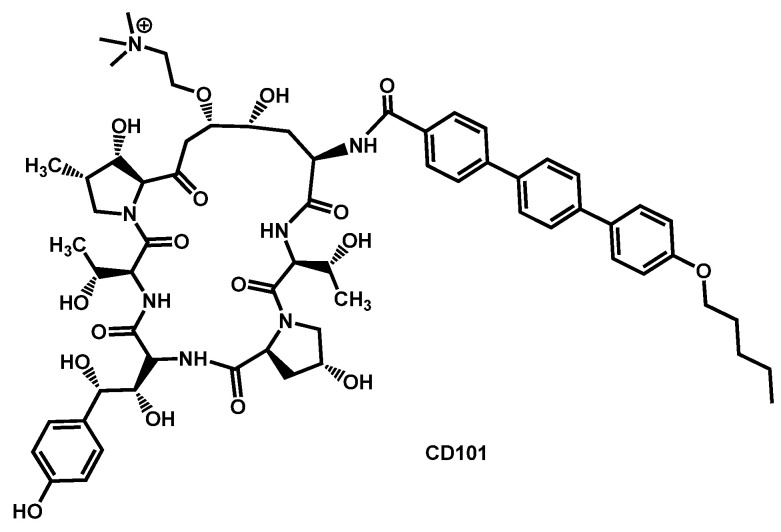
In 2017, CD101 (then named rezafungin) was patented for the treatment of fungal infections (Figure 21) [118]. CD101 is a novel echinocandin with long-acting profile (Figure 21). In 2018, another patent was filed for the use of CD101 alone or in combination with another antifungal drug for the treatment of fungal infections [119]. Overall, CD101 shows encouraging activity against the emerging pathogen C. auris. In late 2017, Berkow and Lockhart reported the activity of CD101 against 100 clinical isolates of C. auris [120].
Figure 21.
Structure of CD101.
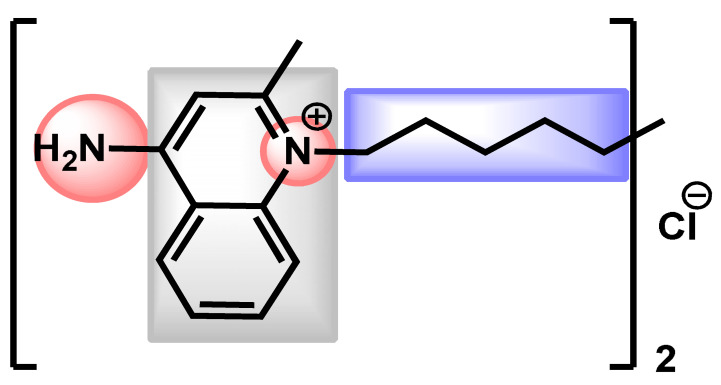
They observed that the MICs values ranged from 0.03 to 8 µg/mL, the average MIC90 was 0.5 µg/mL. In particular, CD101 was investigated against C. auris strains resistant to other echinocandins. The results showed a better activity of CD101 compared to other echinocandins, excepted for isolates containing the S639P amino acid substitution in FKS1 hot spot 1 which is related to previously reported mutations linked to echinocandin resistance in other Candida spp. A few months later, Hager et al. examinated the efficacy of rezafungin (previously CD101) in the treatment of disseminated C. auris infection using an immunocompromised mouse model [121]. Rezafungin proved to have significantly lower average CFU/g of kidney tissue compared with amphotericin B, micafungin and vehicle after ten days of treatment. The pharmacodynamics of rezafungin was then evaluated by Lepak et al. in a neutropenic mouse invasive candidiasis model of C. auris infection [122]. In 2019, Majoros et al. extended the study on CD101 and determined the in vitro susceptibility of 689 clinical isolates of 5 common and 19 rare Candida species, as well as Saccharomyces cerevisiae [123]. Their results proved that rezafungin was an excellent antifungal against common and non-common Candida species, with results similar to other echinocandins excepting caspofungin. In 2020, Helleberg et al. investigated the potency of rezafungin and comparators against 1293 Nordic yeast isolates and 122 Indian C. auris isolates proving that rezafungin had species-specific activity comparable to that of micafungin and anidulafungin. Even if rezafungin was highly active against the majority of common Candida species, it lacked activity against a significant proportion of C. auris isolates with mutations in fks target genes that conferred echinocandin cross-resistance. However, fks1 mutations increased rezafungin MICs notably less than micafungin and anidulafungin MICs in C. auris [124]. Gathering all these results, rezafungin entered a Phase III multicenter, prospective, randomized, double-blind, efficacy and safety clinical trial in 2018 (ClinicalTrials.gov Identifier: NCT03667690) which is still currently recruiting for comparative evaluation of Rezafungin to caspofungin followed by optional oral fluconazole step-down therapy in subjects in subjects with candidemia and/or invasive candidiasis [125].
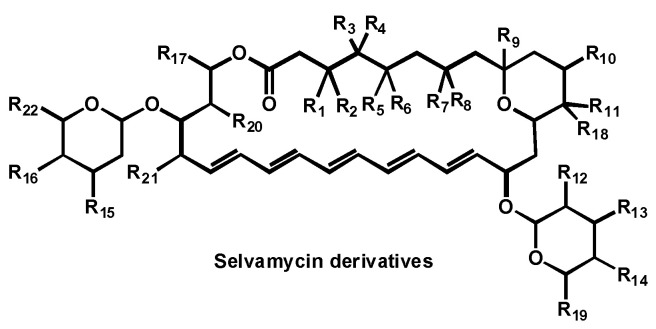
5.3. Selvamycin Analogues
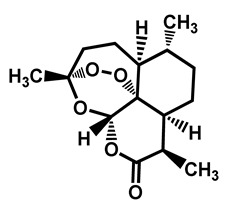
In 2017, a patent, covering selvamicin and its analogues, was filed for the treatment of fungal infections (Figure 22) [126]. Selvamicin resembles the reference antifungals nystatin A1 and amphotericin B, but bears several distinctive structural features, impacting its pharmacokinetics and cell target. The data compiled in the patent showed that selvamicin and its analogues, compared to nystatin A1, are potent antifungal against Candida strains, including C. auris.
Figure 22.
Structure of selvamycin derivatives.
5.4. Polymers
Recently, Arias et al. reported the in vitro and in vivo activity of chitosan against planktonic and sessile forms of C. auris. In a galleria mellonella model of C. auris infection, chitosan decreased the killing effects of C. auris infection without any toxicity to the larvae. The putative mode of action of chitosan could be mediated by the expression of a stress-like gene expression response, conducting to protection in the larvae [127].
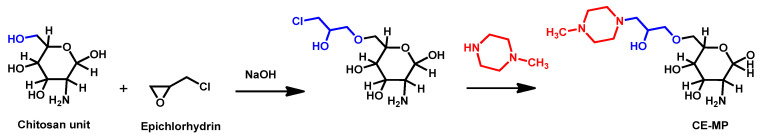
However, in 2017, Chauhan and Loonker described the modification of chitosan to integrate N-methyl piperazine moieties and the biological evaluation of the resulting polymer called CE-MP [128]. They realized the cross-linkage of the naturally occurring linear polysaccharide matrix of chitosan with epichlorohydrin and then incorporated N-methyl piperazine moieties (Scheme 6). The resulting artificial polymer was fully characterized and proved to be active against some fungi but unfortunately displayed no activity against C. auris.
Scheme 6.
Synthesis of CE-MP as reported by Chauhan and Loonker.
Taken together, these results demonstrated that naturally derivated polymers such as chitosan could be interesting alternatives to conventional antifungals.
5.5. Polyclonal Antibody
In 2018, Bujdakova et al. reported the activity of anti-CR3-RP polyclonal antibody against biofilms formed by C. auris [129]. During biofilm formation, Candida species generally expressed the complement receptor 3-related protein (CR3-RP) as surface antigens. The authors proved the presence of CR3-RP in C. auris cells within biofilms and the subsequent activity of anti-CR3-RP polyclonal antibody to eradicate the formation of biofilm by C. auris.
Polymers and polyclonal antibody could represent exciting therapeutic alternatives to small drugs but, for the moment, the number of works dealing with such strategies in the frame of fighting against C. auris is significantly restricted.
6. Traditional Medicines and Natural Compounds
It is notable that the reported data mainly resulted from in vitro evaluation. Few compounds experimented in vivo efficacy, unless specified.
6.1. Small Natural Compounds
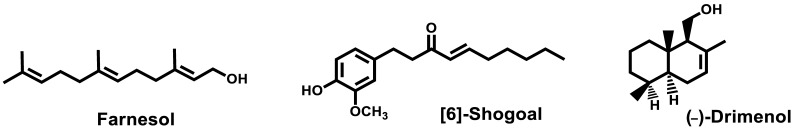
Farnesol, an endogenous quorum sensing molecule, was reported in 2020 by Vartika and Aijaz [130] for its ability to modulate development of biofilms and drug efflux in C. auris. Farnesol also blocked efflux pumps and downregulated biofilm and efflux pump associated genes at a concentration of 125 mM (Figure 23). In parallel, the effects of farnesol were also reported by Kovacs et al. [131,132]. The authors studied the effects of 100–300 µM farnesol on growth, biofilm production ability, production of enzymes related to oxidative stress, triazole susceptibility and virulence towards C. auris strains. After 24 h, farnesol was not able to inhibit the formation of biofilm but caused a significant growth inhibition against C. auris planktonic cells. Moreover, farnesol decreased the metabolic activity and increased the production of ROS. Used in combination with other antifungals, farnesol displayed a synergistic behavior. In vivo, using an immunocompromised murine model of disseminated candidiasis, daily 75 µM farnesol proved to lower the fungal burden and could be a benefit as therapeutics or adjuvant for the treatment of candidiasis.
Figure 23.
Structures of farnesol, [6]-shogoal and (−)-drimenol.
[6]-Shogoal, one of the pungent constituents of ginger, exhibited antifungal and antibiofilm formation effects against C. auris [133]. The mode of action of [6]-shogoal was experimentally related to the reduction of the levels of aspartyl proteinases and downregulation of the expression of the efflux pump-related CDR1-gene in C. auris. However, the in vitro activity displayed by [6]-shogoal, even higher than fluconazole, is quite modest with MIC80 ranging from 32 to 64 µg/mL.
Eight drimane sesquiterpenoids including (−)-drimenol and (+)-albicanol were synthesized from (+)-sclareolide and evaluated for their antifungal activities. (−)-Drimenol (Figure 23) proved to limit the growth of C. auris better than fluconazole in the same concentration [134]. This natural compound also protected C. elegans from death in a candiasis model [134].
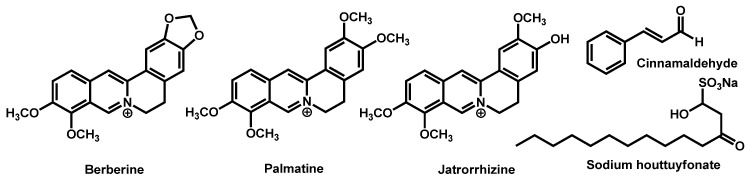
Traditional herbal monomers (THMs), mainly composed of sodium houttuyfonate (SH), berberine (BER), palmatine (PAL), jatrorrhizine (JAT), and cinnamaldehyde (CIN), were evaluated for their potential to induce cell wall modulations in C. auris (Figure 24) [135]. The authors proved that combination of these herbal monomers induced good antifungal activity against C. auris isolates the most potent combinations being SH/CIN and BER/PAL/JAT.
Figure 24.
Composition of the five most common traditional herbal monomers as stated by Liu et al.
Bark and leaf essential oils from Cinnamomum zeylanicum proved in vitro antifungal activity against C. auris, by damaging the membrane and blocking the hyphae formation. From a chemical point of view, the main components of C. zeylanicum leaf is eugenol (62%) and C. zeylanicum bark is trans-cinnamaldehyde (66%), the other components being present at less than 7% [136].
Even if the use of natural compounds is of great interest, the activity of farnesol, [6]-shogoal, (−)-drimenol or common traditional herbal monomers is quite low compared to synthetic drugs and they have to be envisioned as adjuvants or platform molecules for drug chemical development.
6.2. Extracts of Natural Organisms
Marine sponges are among the richest sources of bioactive ingredients from marine organisms. In 2017, Hasaballah et al. reported the biological evaluation of crude extracts of marine sponges, Negombata magnifica and Callyspongia siphonella towards a large panel of biological targets, including C. auris. In addition to their high insecticidal capacity, the two sponge extracts also displayed high antibacterial and antifungal activities, with growth inhibition of 15.3 ± 1.2 mm for N. magnifica and 13.7 ± 1.5 mm for C. siphonella compared to 19.8 ± 0.63 mm for amphotericin B [137].
In 2020, Zhang et al. reported the identification of turbinmicin, a lead antifungal which was identified from marine microbiome (Figure 25). Turbinmicin is highly interesting because it functions through a fungal-specific mode of action, targeting Sec14 of the vesicular trafficking pathway [138]. Moreover, the same team reported the striking ability of turbinmicin to block biofilm formation. Indeed, turbinmicin acts by disruption of extracellular vesicle delivery during biofilm growth [139]. Turbinmicin revealed as a promising anti-biofilm and antifungal drug [140].
Figure 25.
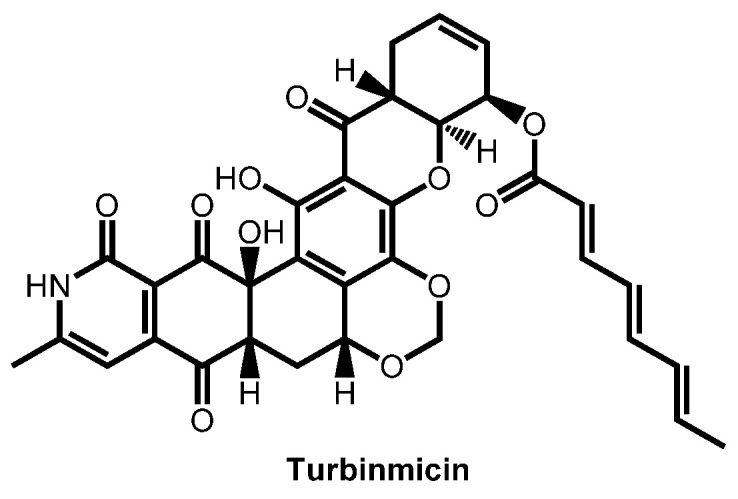
Structure of turbinmicin.
6.3. Peptides and Derivatives
In 2015, Cm-p5, an antifungal hydrophilic peptide derived from the coastal mollusk cenchritis muricatus was reported by Lopez-Abarrategi et al. [141]. Cm-p5 proved to have a fungistatic activity against C. albicans with MIC around 10 µg/mL and moderate toxicity toward mammalian cell lines. Following this first report, the same team obtained mutants of Cm-p5 and then designed and synthetized a helical-stabilized, cyclic, and nontoxic analogue of Cm-p5. This analogue displayed moderate antifungal activity against C. auris [142]. In 2020, Rosenau et al. also reported that the same derivatives of the Cm-p5 were able to inhibit the development of C. auris biofilms in vitro [143].
Ramachandran et al. evaluated the efficacy of three novel cyclic lipopeptides of the class Bacillomycin to limit fungal infection [144]. The lipopeptide analogues, bearing the same peptide sequence Asn-Pro-Tyr-Asn-Gln-Thr-Ser, were purified from a cell-free supernatant of Bacillus subtilis RLID 12.1. The three active lipopeptides exhibited MICs from 3.5 to 8.5 µg/mL against 10 C. auris isolates.
Antimicrobial peptides (AMPs) constitute a key component of these innate immune defences and AMPs from a variety of sources have shown potent antifungal activities [145,146,147,148]. Since they induce no or minimal MDR in target fungi, AMPs are considered strongly attractive as potential therapeutic drugs [149]. Pathirana et al. have investigated salivary Histatin 5 (Hst 5) for its ability to limit fungal infections [150]. Hst 5 is a well-studied salivary cationic peptide. After treatment at a 7.5 µM dose, 55 to 90% of all C. auris cells were killed by Hst 5, irrespective of the MDR-profile strains. The mode of action of Hst 5 is based on its translocation to the cytosol and vacuole and the reported study suggested that it differed from the one of fluconazole. Baso, Garcia et al. recently reported the antifungal properties of θ-defensins, macrocyclic peptides expressed in tissues of old world monkeys [151]. The mode of action of Rhesus θ-defensin 1 (RTD-1), the prototype θ-defensin, occurred by cell permeabilization, correlated with ATP release and intracellular accumulation of killing reactive oxygen species. Other natural defensins isoforms were evaluated and the most promising proved to be more active than caspofungin and/or amphotericin B caspofungin against fluconazole-resistant organisms, including C. auris. θ-defensin activity was compared with Hst 5 and proved to be 200-fold more active and more stable to proteases.
Dal Mas et al. described the effect of Crotamine, a natural antimicrobial peptide (AMP) isolated from a South American rattlesnake on C. auris [152]. Crotamine, which has structural similarities with human defensins, exhibited in vitro activity against most isolates tested, whereas these Candida isolates showed resistance to amphotericin B and fluconazole.
Cathelicidins are a class of epithelial antimicrobial peptides that are expressed in the intestinal epithelium and kill microorganisms by membrane disruption. Haagsman et al. recently obtained cathelicidin-inspired antimicrobial peptides and evaluated their antifungal capacity [153]. These novel antimicrobial peptides termed ‘PepBiotics’, containing between 21 and 37 residues, have been patented and showed a strong inhibitory activity against a large panel of fungi and yeasts at low concentrations (≤1 μM). In particular, they exhibited MIC = 0.6–1.3 µM against C. auris strains.
6.4. Bioinspiration
With the goal to mimic AMPs, Savage et al. recently developed ceragenins (CSAs), a class of non-peptide molecules, based on a common bile acid, that conserve the amphiphilic morphology common to AMPs (Figure 26). They have then evaluated the susceptibilities of C. auris isolates, in biofilm and planktonic forms, to CSAs. In addition, they also measured the effectiveness of the most promising CSAs in gel and cream formulations in treated infected tissue explants [154]. Lead CSAs led to activity comparable with reference drugs and no cross-resistance was observed. In ex-vivo mucosal tissues, CSAs were able to significantly reduce the fungal burden with 2% topical application.
Figure 26.
Structures of selected ceragenins CSAs.
6.5. Necrotrophic Mycoparasitism
Necrotrophic mycoparasitism describes the ability of a fungal species to kill other fungi [155]. In 2018, Junker et al. examinated the use of a predatory yeast, Saccharomycopsis schoenii, as a potential biocontrol agent against C. auris [156]. They investigated the interaction between the two species by seeding equal quantity of dimorphic S. schoenii and ovoid C. auris NCPF8985#20 cells on minimal media agar on microscopy slides. Using specialized penetration pegs, S. schoenii attacked C. auris cells upon contact and killed 34% of them within a period of 6 h. All isolates of Candida species tested, including several drug resistant C. auris strains, were susceptible to predation by S. schoenii, opening new possibilities to eradicate such multidrug resistant strains.
7. Metal, Metal Complexes and Metalloids
Recently, Goldman et al. evaluated the potential of gallium, already known for its antibacterial activity, as antifungal [157]. They demonstrated that gallium nitrate had a fungistatic action against fungi. Regarding C. auris, several strains (473/2015, 490/2015, 501/2015, and 502/2015) were completely inhibited by gallium at concentrations of 128–256 mg/L. However, others (strains 467/2015, 470/2015, and 484/2015) survived the challenge at the higher concentration tested limiting the use of gallium as a broad-spectrum antifungal agent.
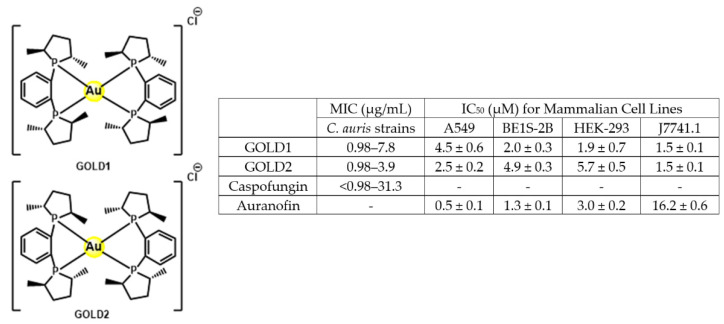
In 2020, the team of Garneau-Tsodikova and Awuah explored the potential of linear and square-planar gold(I)−phosphine complexes as antimicrobial agents against a panel of 28 fungal strains [158]. Compounds GOLD1 and GOLD2 proved to be the most efficient against C. auris, with MIC similar to caspofungin. Moreover, these two gold-complexes displayed low cytotoxicity against a panel of cell lines (Figure 27).
Figure 27.
Gol(I)-complexes as antifungals: structure, MIC values range and cytotoxicity.
The amount of data gathered on the use of metal and metallic complexes towards C. auris is too low to ascertain the interest of such strategy, but the first data are encouraging. Therefore, medicinal chemists should go further with this alternative area of research in the fight against fungal resistant strains.
8. Others Approaches
8.1. Probiotics and Postbiotics
The review by Zhang et al. described the recent reports that indicate that probiotics may also contribute to protect against fungal infections [159]. Even if some publications have evaluated the benefits of probiotics against fungal infections caused by C. albicans, C. glabrata, A. fumigatus, T. tonsurans, M. canis, M. gypseum, or E. flocossum, no reports on C. auris are presented in that review published in 2019.
Late 2019, Rao et al. exploited the probiotic properties of two novel, food-derived yeasts, Saccharomyces cerevisiae (strain KTP) and Issatchenkia occidentalis (strain ApC). These alternative approaches to combat widespread opportunistic fungal infections proved to be effective to inhibit virulence traits such as adhesion, biofilm formation and filamentation of Candida spp., especially C. auris [160,161].
In 2020, Campos-Junqueira et al. described the pro- and postbiotic activity of Lactobacillus paracasei 28.4 against C. auris. It is notable that they conducted an in vivo study with G. mellonella larvae infected with C. auris. Injections of LPCE and LPF1 (crude extract and fraction 1 derived from L. paracasei 28.4 supernatant, respectively) prolonged survival of these insects compared to a control group (p < 0.05) and modulated the host immune response [162].
8.2. Nanoparticles and Coatings
In 2019, Philip and Kuriakose reported the synthesis of superparamagnetic Fe2O3 nanoparticles stabilized by biocompatible supramolecular β-cyclodextrin and their evaluation against three strains of C. auris but with a moderate MIC = 500 µg/mL [163].
Chapman, Truong et al. reported in 2020 the fabrication of long-term microbicidal silver nanoparticle clusters [164]. This silver nanoparticle cluster coating was constructed on copper surface using an ion-exchange and reduction reaction. The resulting surface was contaminated by C. auris and evaluated at extended periods. After seven days, 90% of C. auris proved to be non-viable on the new designed surface.
In parallel, using a microwave assisted synthetic approach, Lara et al. reported the synthesis of pure round silver nanoparticles (AgNPs) and their use to inhibit C. auris biofilm formation on surfaces [165]. These AgNP-treated biofilms showed cell wall damage mostly by disruption and distortion of the outer surface of the fungal cell wall. Moreover, the AgNPs-functionalized fibers proved to be stable and kept their fungicidal potential even after repeated thorough washes.
Very recently, Nosanchuck et al. described the effects of nanoparticles capable of generating nitric oxide (NO) to kill C. auris [166]. This NO-nanotherapeutics were incubated with six different C. auris strains and eradicated both planktonic and biofilm C. auris with a 10 mg/mL and emerged as a promising approach to fight against MDR-fungal strains.
Bismuth nanoparticles (BiNPs) demonstrated strong antifungal activity against a panel of C. auris strains, with MIC ranging from 1 to 4 µg/mL. Moderate effects of these nanoantibiotics were also examined by scanning electron microscopy and showed the disruption of the C. auris cell morphology and the biofilm structure [167].
8.3. Hydrogels
In 2019, Shukla and Vera-Gonzalez patented an aspartic protease-triggered antifungal hydrogel able to locally deliver antifungal drugs that specifically respond to aspartic proteases secreted by virulent and pathogenic Candida species [168].
In 2020, Rosenau et al. described a novel anti-infective multicomponent gatekeeper hydrogel [169]. This bilayer hydrogel protected the patient from invasion by C. auris from infected wounds, by combining to complementary layers. The first layer reduced the loading of the pathogen C. auris by selective cell capturing within the affinity layer of the gel followed by subsequent inactivation within the therapeutic gel layer.
In a similar manner, Gupta et al. reported similar antibacterial silver-nanoparticle hydrogel for wound dressing applications [170]. The particularity of their design relied on the green chemistry approach—based on natural curcumin as reducing agent-developed to obtain the nanoparticles.
8.4. Irradiation
Candida spp. are the most common cause of fungal infections worldwide and the fifth most common cause of nosocomial infections [171]. In particular, C. auris resists disinfection with cleaning agents widely used in hospitals and long-term care facilities [31,172], is frequently drug resistant, and can persist in the environment for months [173]. As a consequence, new protocols to identify how to eliminate C. auris from surface and devices drove several studies.
In 2018, Cadnum et al. stated about the relative resistance of C. auris to be eradicated by ultraviolet light [174]. Before this study, it was not clear if mobile ultraviolet-C (UV-C) light room decontamination devices efficiently cleaned healthcare facilities. From their data, it appeared that C. auris was significantly less susceptible to killing by UV-C than methicillin-resistant Staphylococcus aureus. This first statement brought to light the need in understanding how efficiently eradicate C. auris using UV lights. Consequently, in 2019, Ponnanchan et al. evaluated the antifungal activity of ultraviolet C (UVC) light using mercury vapour lamp with a peak emission of 254 ± 2 nm against 32 C. auris isolates. Contaminated plates were held parallel to the UVC source throughout the period of exposure. After 15 min of irradiation at this wavelength, all experiments demonstrated complete inhibition of growth up to 72 h incubation for C. auris [35].
In a parallel manner, in 2019, Maslo et al. reported the efficacy of pulsed-xenon ultraviolet light to eradicate C. auris [175]. After inoculation, each sample was exposed during uninterrupted periods of 5, 10 or 15 min at distances ranging from 1 to 2 m. The PX-UV mobile device killed 99.4% of C. auris CFU on the surface after 5 min irradiation at 1 m-distance. The time and distance of exposure have also been quantified by de Groot et al. [176]. The optimum for C. auris eradication was obtained after 30 min of decontamination at 2 m. Dividing time by two or double the distance decreased the activity by 10- and 50-fold, respectively.
In 2020, Lemons et al. also demonstrated the efficiency of ultraviolet irradiation to kill C. auris [177]. Ultraviolet germicidal irradiation (UVGI) was investigated to inactivate ten clinically relevant strains of C. auris. In order to determine dose-response curves, each sample was exposed eleven times to range of UV-dose from 10 to 150 mJ/cm2. From these data, C. auris required more energy than C. albicans to be eradicated. As a consequence, the authors stated that UVGI could be applied against C. auris but the variation of susceptibility would have to be considered.
From these reports, irradiation could be a powerful tool to eradicate C. auris in medical area. However, all parameters should be carefully estimated to avoid a lack of effectiveness and propose tailor-made solutions to eradicate C. auris.
9. Conclusions and Perspectives
Through gathering all these data it appeared that the fight against C. auris is continuously increasing for the last five years, in parallel of its emergence and definition as a fungus of concern. Even if most reports dealt with Candida species, some important works focused only on C. auris and took in consideration its own particularities. No perfect solution has been stated for the moment, but clear opportunities have been demonstrated whether from drug repositioning, combination of drugs, or the design of novel antifungal entities. From a medicinal chemist point of view, some molecular features seemed to emerge as required for the design of potent antifungals against C. auris, and there is no doubt that these preferred moieties should inspire the conception of novel effective drugs. As always, nature is a great provider of active compounds and bioinspiration also drives some exciting research works. Even if it is of great importance to treat patients, it is also absolutely required to clean devices and surfaces to avoid contamination and proliferation. Therefore, the emerging solutions based on the specific characteristic of C. auris in terms of irradiation or antimicrobial surfaces are of particular interest. Such an approach could be considered as tailor-made conception towards C. auris. To summarize, ongoing works are very encouraging, but there is still an urgent need to propose therapeutic alternatives to eradicate C. auris.
Author Contributions
Conceptualization: M.B., S.J., S.H. and Z.F.; writing—original draft preparation, M.B. and S.J.; writing—review and editing, S.H. and Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by the Agence Nationale de la Recherche (ANR) in the setting of project “InnateFun”, promotional reference ANR-16-IFEC-0003-05, in the “Infect-ERA” program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti A., Singh S. Multidrug-resistant Candida auris: An epidemiological review. Expert Rev. Anti-Infect. Ther. 2020;18:551–562. doi: 10.1080/14787210.2020.1750368. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes J., Fisher M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019;52:84–89. doi: 10.1016/j.mib.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz Gaitán A.C., Moret A., López Hontangas J.L., Molina J.M., Aleixandre López A.I., Hernández Cabezas A., Mollar Maseres J., Arcas R.C., Gómez Ruiz M.D., Chiveli M.A., et al. Nosocomial fungemia by Candida auris: First four reported cases in continental Europe. Rev. Iberoam. Micol. 2017;34:23–27. doi: 10.1016/j.riam.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Public Health England Research and Analysis: Candida auris Identified in England. [(accessed on 18 September 2020)];2017 Available online: https://www.gov.uk/government/publications/candida-auris-emergence-in-england/candida-auris-identified-in-england.
- 6.ECDC Candida in Healthcare Settings. [(accessed on 18 September 2020)];2016 :8. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Candida-in-healthcare-settings_19-Dec-2016.pdf.
- 7.Kim M.N., Shin J.H., Sung H., Lee K., Kim E.-C., Ryoo N., Lee J.-S., Jung S.-I., Park K.H., Kee S.J., et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: Identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 2009;48:e57–e61. doi: 10.1086/597108. [DOI] [PubMed] [Google Scholar]
- 8.Lee W.G., Shin J.H., Uh Y., Kang M.G., Kim S.H., Park K.H., Jang H.C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011;49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A., Sharma C., Duggal S., Agarwal K., Prakash A., Singh P.K., Jain S., Kathuria S., Randhawa H.S., Hagen F., et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013;19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakrabarti A., Sood P., Rudramurthy S.M., Chen S., Kaur H., Capoor M., Chhina D., Rao R., Eshwara V.K., Xess I., et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhary A., Anil Kumar V., Sharma C., Prakash A., Agarwal K., Babu R., Dinesh K.R., Karim S., Singh S.K., Hagen F., et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 12.Magobo R.E., Corcoran C., Seetharam S., Govender N.P. Candida auris-associated candidemia, South Africa. Emerg. Infect. Dis. 2014;20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emara M., Ahmad S., Khan Z., Joseph L., Al-Obaid I., Purohit P., Bafna R. Candida auris candidemia in Kuwait, 2014. Emerg. Infect. Dis. 2015;21:1091–1092. doi: 10.3201/eid2106.150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arensman K., Miller J.L., Chiang A., Mai N., Levato J., LaChance E., Anderson M., Beganovic M., Dela Pena J. Clinical Outcomes of Patients Treated for Candida auris Infections in a Multisite Health System, Illinois, USA. Emerg. Infect. Dis. 2020;26:876–880. doi: 10.3201/eid2605.191588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J.Y., Bradley N., Brooks S., Burney S., Wassner C. Management of Patients with Candida auris Fungemia at Community Hospital, Brooklyn, New York, USA, 2016-20181. Emerg. Infect. Dis. 2019;25:601–602. doi: 10.3201/eid2503.180927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow N.A., Gade L., Tsay S.V., Forsberg K., US Candida auris Investigation Team Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: A molecular Candida auris in contemporary mycology labs at epidemiological survey. Lancet Infect. Dis. 2018;18:1377–1384. doi: 10.1016/S1473-3099(18)30597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo B., Melo A.S.A., Perozo-Mena A., Hernandez M., Francisco E.C., Hagen F., Meis J.F., Colombo A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Taori S.K., Khonyongwa K., Hayden I., Dushyanthie G.I.D., Athukorala A.D., Letters A., Fife A., Desai N., Borman A.M. Candida auris outbreak: Mortality, interventions and cost of sustaining control. J Infect. 2019;79:601–611. doi: 10.1016/j.jinf.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Clancy C.J., Nguyen M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2017;64:141–143. doi: 10.1093/cid/ciw696. [DOI] [PubMed] [Google Scholar]
- 20.Sears D., Schwartz B.S. Candida auris: An emerging multidrug-resistant pathogen. Int. J. Infect. Dis. 2017;63:95–98. doi: 10.1016/j.ijid.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Sabino R., Verissimo C., Pereira A.A., Antunes F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms. 2020;8:181. doi: 10.3390/microorganisms8020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., Colombo A.L., Calvo B., Cuomo C.A., Desjardins C.A., et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pristov K.E., Ghannoum M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infection. 2019;25:792–798. doi: 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Munoz J.F., Gade L., Chow N.A., Loparev V.N., Juieng P., Berkow E.L., Farrer R.A., Litvintseva A.P., Cuomo C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018;9:5346–5358. doi: 10.1038/s41467-018-07779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow N.A., de Groot T., Badali H., Abastabar M., Chiller T.M., Meis J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019;25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desoubeaux G., Bailly E., Guillaume C., De Kyvon M.A., Tellier A.C., Morange V., Bernard L., Salame E., Quentin R., Chandenier J. Candida auris in contemporary mycology labs: A few practical tricks to identify it reliably according to one recent French experience. J. Mycol. Med. 2018;28:407–410. doi: 10.1016/j.mycmed.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller M.A., Diekema D.J., Turnidge J.D., Castanheira M., Jones R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. Open Forum Infect. Dis. 2019;6(Suppl. S1):S79–S94. doi: 10.1093/ofid/ofy358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson B.R., Chow N., Forsberg K., Litvintseva A.P., Lockhart S.R., Welsh R., Vallabhaneni S., Chiller T. On the Origins of a Species: What Might Explain the Rise of Candida auris? J. Fungi. 2019;5:58. doi: 10.3390/jof5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadevall A., Kontoyiannis D.P., Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio. 2019;10:e01397-19. doi: 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kean R., Sherry L., Townsend E., McKloud E., Short B., Akinbobola A., Mackay W.G., Williams C., Jones B.L., Ramage G. Surface disinfection challenges for Candida auris: An in-vitro study. J. Hosp. Infect. 2018;98:433–436. doi: 10.1016/j.jhin.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Yue H., Bing J., Zheng Q., Zhang Y., Hu T., Du H., Wang H., Huang G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018;7:188. doi: 10.1038/s41426-018-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentz M.L., Sexton D.J., Welsh R.M., Litvintseva A.P. Phenotypic switching in newly emerged multidrug-resistant patho, gen Candida auris. Med. Mycol. 2018;57:636–638. doi: 10.1093/mmy/myy100. [DOI] [PubMed] [Google Scholar]
- 33.Kvaal C., Lachke S.A., Srikantha T., Daniels K., McCoy J., Soll D.R. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 1999;67:6652–6662. doi: 10.1128/IAI.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvaal C.A., Srikantha T., Soll D.R. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect. Immun. 1997;65:4468–4475. doi: 10.1128/IAI.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaffin W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Bing J., Zheng Q., Zhang F., Liu J., Yue H., Tao L., Du H., Wang Y., Wang H., et al. The first isolate of Candida auris in China: Clinical and biological aspects. Emerg. Microbes Infect. 2018;7:93. doi: 10.1038/s41426-018-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar D., Banerjee T., Pratap C.B., Tilak R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J. Infect. Dev. Ctries. 2015;9:435–437. doi: 10.3855/jidc.4582. [DOI] [PubMed] [Google Scholar]
- 38.Ghannoum M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000;13:122–143. doi: 10.1128/CMR.13.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkin E., Hager C., Chandra J., Mukherjee P.K., Retuerto M., Salem I., Long L., Isham N., Kovanda L., Borroto-Esoda K.-, et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017;61:e02396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak A.P., Green B.J., Beezhold D.H. Fungal hemolysins. Med. Mycol. 2013;51:1–16. doi: 10.3109/13693786.2012.698025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira A.V., Prado C.G., Carvalho R.R., Dias K.S., Dias A.L. Candida albicans and non-C. albicans Candida species: Comparison of biofilm production and metabolic activity in biofilms, and putative virulence properties of isolates from hospital environments and infections. Mycopathologia. 2013;175:265–272. doi: 10.1007/s11046-013-9638-z. [DOI] [PubMed] [Google Scholar]
- 42.Day A.M., McNiff M.M., da Silva Dantas A., Gow N.A.R., Quinn J. Hog1 Regulates Stress Tolerance and Virulence in the Emerging Fungal Pathogen Candida auris. mSphere. 2018;3:e00506-18. doi: 10.1128/mSphere.00506-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee S., Alampalli S.V., Nageshan R.K., Chettiar S.T., Joshi S., Tatu U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genom. 2015;16:686. doi: 10.1186/s12864-015-1863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma C., Kumar N., Pandey R., Meis J.F., Chowdhary A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect. 2016;13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherry L., Ramage G., Kean R., Borman A., Johnson E.M., Richardson M.D., Rautemaa-Richardson R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017;23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S., Uppuluri P., Mamouei Z., Alqarihi A., Elhassan H., French S., Lockhart S.R., Chiller T., Edwards J.E., Jr., Ibrahim A.S. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019;15:e1007460. doi: 10.1371/journal.ppat.1007460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pushpakom S., Iorio F., Eyers P., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 48.Talevi A., Bellera C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discov. 2020;15:397–401. doi: 10.1080/17460441.2020.1704729. [DOI] [PubMed] [Google Scholar]
- 49.Wall G., Chaturvedi A.K., Wormley F.L., Jr., Lopez-Ribot J.L., Wiederhold N.P., Patterson H.P., Patterson T.F. Screening a Repurposing Library for Inhibitors of Multidrug-Resistant Candida auris Identifies Ebselen as a Repositionable Candidate for Antifungal Drug Development. Antimicrob. Agents Chemother. 2018;62:e01084-18. doi: 10.1128/AAC.01084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamouei Z., Alqarihi A., Singh S., Xu S., Mansour M.K., Ibrahim A.S., Uppuluri P. Alexidine Dihydrochloride Has Broad-Spectrum Activities against Diverse Fungal Pathogens. mSphere. 2018;3:e00539-18. doi: 10.1128/mSphere.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Oliveira H.C., Monteiro M.C., Rossi S.A., Pemán J., Ruiz-Gaitán A., Mendes-Giannini M., Mellado E., Zaragoza O. Identification of Off-Patent Compounds That Present Antifungal Activity Against the Emerging Fungal Pathogen Candida auris. Frontiers Cell. Infect. Microbiol. 2019;9:83. doi: 10.3389/fcimb.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gowri M., Jayashree B., Jeyakanthan J., Girija E.K. Sertraline as a promising antifungal agent: Inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J. Appl. Microbiol. 2020;128:426–437. doi: 10.1111/jam.14490. [DOI] [PubMed] [Google Scholar]
- 53.Diluccio R., Reidenberg B. Methods and pharmaceutical compositions for treating Candida auris in blood comprising administering taurolidine derivatives. WO 2019126695 A2 20190627. U.S. Patent 16/229,898. PCT Int. Appl. (2019) 2019 Jun 27;
- 54.Hübner N.O., Siebert J., Kramer A. Octenidine Dihydrochloride, a Modern Antiseptic for Skin, Mucous Membranes and Wounds. Skin Pharmacol. Physiol. 2010;23:244–258. doi: 10.1159/000314699. [DOI] [PubMed] [Google Scholar]
- 55.Ponnachan P., Vinod V., Pullanhi U., Varma P., Singh S., Biswas R., Kumar A. Antifungal activity of octenidine dihydrochloride and ultraviolet-C light against multidrug-resistant Candida auris. J Hosp Infect. 2019;102:120–124. doi: 10.1016/j.jhin.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Agnew-Francis K.A., Tang Y., Lin X., Low Y.S., Wun S.J., Kuo A., Elias S., Lonhienne T., Condon N.D., Pimentel B., et al. Herbicides That Target Acetohydroxyacid Synthase Are Potent Inhibitors of the Growth of Drug-Resistant Candida auris. ACS Infect. Dis. 2020;6:2901–2912. doi: 10.1021/acsinfecdis.0c00229. [DOI] [PubMed] [Google Scholar]
- 57.Wall G., Herrera N., Lopez-Ribot J.L. Repositionable compounds with antifungal activity against multidrug resistant Candida auris identified in the medicines for malaria venture’s pathogen box. J. Fungi. 2019;5:92. doi: 10.3390/jof5040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y., Totten M., Memon W., Ying C., Zhang S.X. In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen Candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 2020;64:e02063. doi: 10.1128/AAC.02063-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barreto T.L., de Freitas A.L.D., Ishida K., Rossato L., Colombo A.L., Meis J.F., Lopes L.B. Miltefosine as an alternative strategy in the treatment of the emerging fungus Candida auris. Int. J. Antimicrob. Agents. 2020:106049. doi: 10.1016/j.ijantimicag.2020.106049. [DOI] [PubMed] [Google Scholar]
- 60.Singh S., Uppuluri P., Alqarihi A., Elhassan H., French S., Lockhart S.R., Chiller T., Edwards J.E., Jr., Ibrahim A.S. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. bioRxiv Microbiol. 2018:1–54. doi: 10.1101/465096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J.F., Badali H. In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2017;61:e01056-17. doi: 10.1128/AAC.01056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz P., Bidaud A.L., Dannaoui E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci. Rep. 2020;10:21448. doi: 10.1038/s41598-020-78588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien B., Chaturvedi S., Chaturvedi V. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob. Agents Chemother. 2020;64:e02195. doi: 10.1128/AAC.02195-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bidaud A.L., Botterel F., Chowdhary A., Dannaoui E. In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob. Agents Chemother. 2019;63:e01393. doi: 10.1128/AAC.01393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eldesouky H.E., Li X., Abutaleb N.S., Mohammad H., Seleem M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents. 2018;52:754–761. doi: 10.1016/j.ijantimicag.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Eldesouky H.E., Salama E.A., Li X., Hazbun T.R., Mayhoub A.S., Seleem M.N. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci. Rep. 2020;10:7525. doi: 10.1038/s41598-020-64571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahmoudi S., Rezaie S., Daie Ghazvini R., Hashemi S.J., Badali H., Foroumadi A., Diba K., Chowdhary A., Meis J.F., Khodavaisy S. In Vitro Interaction of Geldanamycin with Triazoles and Echinocandins against Common and Emerging Candida Species. Mycopathologia. 2019;184:607–613. doi: 10.1007/s11046-019-00370-7. [DOI] [PubMed] [Google Scholar]
- 68.Bidaud A.L., Djenontin E., Botterel F., Chowdhary A., Dannaoui E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents. 2020;55:105901. doi: 10.1016/j.ijantimicag.2020.105901. [DOI] [PubMed] [Google Scholar]
- 69.Eldesouky H.E., Lanman N.A., Hazbun T.R., Seleem M.N. Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence. 2020;11:1466–1481. doi: 10.1080/21505594.2020.1838741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eldesouky H.E., Salama E.A., Lanman N.A., Hazbun T.R., Seleem M.N. Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 2020;65:e00684-20. doi: 10.1128/AAC.00684-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Revie N.M., Robbins N., Whitesell L., Frost J.R., Appavoo S.D., Yudin A.K., Cowen L.E. Oxadiazole-containing macrocyclic peptides potentiate azole activity against pathogenic Candida species. mSphere. 2020;5:e00256-20. doi: 10.1128/mSphere.00256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iyer K.R., Camara K., Daniel-Ivad M., Trilles R., Pimentel-Elardo S.M., Fossen J.L., Marchillo K., Liu Z., Singh S., Muñoz J.F., et al. An oxindole efflux inhibitor potentiates azoles and impairs virulence in the fungal pathogen Candida auris. Nat. Commun. 2020;11:6429. doi: 10.1038/s41467-020-20183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eldesouky H.E., Salama E.A., Hazbun T.R., Mayhoub A.S., Seleem M.N. Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Sci. Rep. 2020;10:6089. doi: 10.1038/s41598-020-62976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaban S., Patel M., Ahmad A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020;10:1162. doi: 10.1038/s41598-020-58203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia C., Burgain A., Chaillot J., Pic É., Khemiri I., Sellam A. A phenotypic small-molecule screen identifies halogenated salicylanilides as inhibitors of fungal morphogenesis biofilm formation and host cell invasion. Sci. Rep. 2018;8:11559. doi: 10.1038/s41598-018-29973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Genberg C., Beus C.S., Savage P.B. Methods for treating fungal infections using a cationic steroid antimicrobial. WO 2018204506 A1 20181108. PCT Int. Appl. (2018) 2018 Nov 8;
- 77.Tetz G., Collins M., Vikina D., Tetz V. In Vitro Activity of a Novel Antifungal Compound MYC-053 against Clinically Significant Antifungal-Resistant Strains of Candida glabrata Candida auris Cryptococcus neoformans and Pneumocystis spp. Antimicrob. Agents Chemother. 2019;63:e01975-18. doi: 10.1128/AAC.01975-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tetz G., Collins M., Vikina D., Tetz V. In vitro activity of a novel antifungal compound MYC-053 against clinically significant antifungal-resistant strains of Candida glabrata Candida auris Cryptococcus neoformans and Pneumocystis spp. bioRxiv Microbiol. 2018:1–24. doi: 10.1101/409896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamdy R., Fayed B., Hamoda A.M., Rawas-Qalaji M., Haider M., Soliman S.S.M. Essential Oil-Based Design and Development of Novel Anti-Candida Azoles Formulation. Molecules. 2020;25:1463. doi: 10.3390/molecules25061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montoya M.C., Beattie S., Alden K.M., Krysan D.J. Derivatives of the antimalarial drug mefloquine are broad spectrum antifungal molecules with activity against drug-resistant clinical isolates. Antimicrob. Agents Chemother. 2020;64:e02331-19. doi: 10.1128/AAC.02331-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orofino F., Truglio G.I., Fiorucci D., D’Agostino I., Borgini M., Poggialini F., Zamperini C., Dreassi E., Maccari L., Torelli R., et al. In vitro characterization ADME analysis and histological and toxicological evaluation of BM1 a macrocyclic amidinourea active against azole-resistant Candida strains. Int. J. Antimicrob. Agents. 2020;55:105865. doi: 10.1016/j.ijantimicag.2019.105865. [DOI] [PubMed] [Google Scholar]
- 82.Hagras M., Salama E.A., Sayed A.M., Abutaleb N.S., Kotb A., Seleem M.N., Mayhoub A.S. Oxadiazolylthiazoles as novel and selective antifungal agents. Eur. J. Med. Chem. 2020;189:112046. doi: 10.1016/j.ejmech.2020.112046. [DOI] [PubMed] [Google Scholar]
- 83.Al-Trawneh S.A., Al-Dawdieh S.A., Abutaleb N.S., Tarawneh A.H., Salama E.A., El-Abadelah M.M., Seleem M.N. Synthesis of new pyrazolo[5-1-c][1-2-4]triazines with antifungal and antibiofilm activities. Chem. Pap. 2020;74:1241–1252. doi: 10.1007/s11696-019-00974-9. [DOI] [Google Scholar]
- 84.Argomedo L., Barroso V.M., Barreiro C.S., Darbem M.P., Ishida K., Stefani H.A. Novel 2-Aryloxazoline Compounds Exhibit an Inhibitory Effect on Candida spp. Including Antifungal-Resistant Isolates. ACS Med. Chem. Lett. 2020;11:2470–2475. doi: 10.1021/acsmedchemlett.0c00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pamarthi R., Kumar R., Sankara C.S., Lowe G.J., Zuegg J., Singh S.K., Ganesh M. α-Iodonitroalkenes as Potential Antifungal and Antitubercular Agents. ChemistrySelect. 2020;5:12272. doi: 10.1002/slct.202003251. [DOI] [Google Scholar]
- 86.Thamban Chandrika N., Dennis E.K., Brubaker K.R., Kwiatkowski S., Watt D.S., Garneau-Tsodikova S. Broad-Spectrum Antifungal Agents: Fluorinated Aryl- and Heteroaryl-Substituted Hydrazones. ChemMedChem. 2021;16:124–133. doi: 10.1002/cmdc.202000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitsuyama J., Nomura N., Hashimoto K., Yamada E., Nishikawa H., Kaeriyama M., Kimura A., Todo Y., Narita H. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 2008;52:1318–1324. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nishikawa H., Yamada E., Shibata T., Uchihashi S., Fan H., Hayakawa H., Nomura N., Mitsuyama J. Uptake of T-2307, a novel arylamidine, in Candida albicans. J. Antimicrob. Chemother. 2010;65:1681–1687. doi: 10.1093/jac/dkq177. [DOI] [PubMed] [Google Scholar]
- 89.Wiederhold N.P., Fothergill A.W., McCarthy D.I., Najvar L.K., Bocanegra R., Olivo M., Kirkpatrick W.R., Patterson T.F., Fukuda Y., Mitsuyama J. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob. Agents Chemother. 2015;59:1341–1343. doi: 10.1128/AAC.04228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiederhold N.P., Fothergill A.W., McCarthy D.I., Najvar L.K., Bocanegra R., Olivo M., Kirkpatrick W.R., Patterson T.F., Fukuda Y., Mitsuyama J. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J. Antimicrob. Chemother. 2016;71:692–695. doi: 10.1093/jac/dkv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishikawa H., Sakagami T., Yamada E., Fukuda Y., Hayakawa H., Nomura N., Mitsuyama J., Miyazaki T., Mukae H., Kohno S. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J. Antimicrob. Chemother. 2016;71:1845–1855. doi: 10.1093/jac/dkw095. [DOI] [PubMed] [Google Scholar]
- 92.Shibata T., Takahashi T., Yamada E., Kimura A., Nishikawa H., Hayakawa H., Nomura N., Mitsuyama J. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 2012;56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiederhold N.P., Najvar L.K., Jaramillo R., Olivo M., Patterson H., Connell A., Fukuda Y., Mitsuyama J., Catano G., Patterson T.F. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob. Agents Chemother. 2020;64:e02198-19/1–e02198-19/5. doi: 10.1128/AAC.02198-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berkow E.L., Angulo D., Lockhart S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob. Agents Chemother. 2017;61:e00435-17. doi: 10.1128/AAC.00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCarthy M.W., Walsh T.J. Drug development challenges and strategies to address emerging and resistant fungal pathogens. Expert Rev. Anti. Infect. Ther. 2017;15:577–584. doi: 10.1080/14787210.2017.1328279. [DOI] [PubMed] [Google Scholar]
- 96.Angulo Gonzalez D.A., Barat S.A. Antifungal agents, like ibrexafungerp (SCY-078) for Candida auris decolonization. WO 2020232037 A1 20201119. PCT Int. Appl. (2020) 2020 Nov 19;
- 97.Arendrup M.C., Jørgensen K.M., Hare R.K., Chowdhary A. In Vitro Activity of Ibrexafungerp (SCY-078) against Candida auris Isolates as Determined by EUCAST Methodology and Comparison with Activity against C. albicans and C. glabrata and with the Activities of Six Comparator Agents. Antimicrob. Agents Chemother. 2020;64:e02136-19. doi: 10.1128/AAC.02136-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Y.C., Barat S.A., Borroto-Esoda K., Angulo D., Chaturvedi S., Chaturvedi V. Pan-resistant Candida auris isolates from the outbreak in New York are susceptible to ibrexafungerp (a glucan synthase inhibitor) Int. J. Antimicrob. Agents. 2020;55:105922. doi: 10.1016/j.ijantimicag.2020.105922. [DOI] [PubMed] [Google Scholar]
- 99.Ghannoum M., Isham N., Angulo D., Borroto-Esoda K., Barat S., Long L. Efficacy of Ibrexafungerp (SCY-078) against Candida auris in an In Vivo Guinea Pig Cutaneous Infection Model. Antimicrob. Agents Chemother. 2020;64:e00854-20. doi: 10.1128/AAC.00854-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghannoum M., Arendrup M.C., Chaturvedi V.P., Lockhart S.R., McCormick T.S., Chaturvedi S., Berkow E.L., Juneja D., Tarai B., Azie N., et al. Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics. 2020;9:539. doi: 10.3390/antibiotics9090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chu S., Long L., Sherif R., McCormick T.S., Borroto-Esoda K., Barat S., Ghannoum M.A. A second generation fungerp analog SCY-247, shows potent in vitro activity against Candida auris and other clinically relevant fungal isolates. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.01988-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berkow E.L., Lockhart S.R. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J. Antimicrob. Chemother. 2018;73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 103.Hager C.L., Larkin E.L., Long L., Abidi F.Z., Shaw K.J., Ghannoum M.A. In Vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob. Agents Chemother. 2018;62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arendrup M.C., Chowdhary A., Astvad K.M.T., Joergensen K.M. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob. Agents Chemother. 2018;62:e01225-18/1–e01225-18/9. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Lee M.H., Paderu P., Lee A., Jimenez-Ortigosa C., Park S., Mansbach R.S., Shaw K.J., Perlin D.S. Significantly Improved Pharmacokinetics Enhances In Vivo Efficacy of APX001 against Echinocandin- and Multidrug-Resistant Candida Isolates in a Mouse Model of Invasive Candidiasis. Antimicrob. Agents Chemother. 2018;62:e00425-18. doi: 10.1128/AAC.00425-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao M., Lepak A.J., Andes D.R., VanScoy B., Bader J.C., Ambrose P.G., Marchillo K., Vanhecker J., Andes D.R. In Vivo Pharmacokinetics and Pharmacodynamics of APX001 against Candida spp. in a Neutropenic Disseminated Candidiasis Mouse Model. Antimicrob. Agents Chemother. 2018;62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiederhold N.P., Najvar L.K., Shaw K.J., Jaramillo R., Patterson H., Olivo M., Catano G., Patterson T.F. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob. Agents Chemother. 2019;63:e01120-19. doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arendrup M.C., Chowdhary A., Jørgensen K.M., Meletiadis J. Manogepix (APX001A) In Vitro Activity against Candida auris: Head-to-Head Comparison of EUCAST and CLSI MICs. Antimicrob. Agents Chemother. 2020;64:e00656-20. doi: 10.1128/AAC.00656-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu Y., Kilburn S., Kapoor M., Chaturvedi S., Shaw K.J., Chaturvedi V. In Vitro Activity of Manogepix against Multidrug-Resistant and Panresistant Candida auris from the New York Outbreak. Antimicrob. Agents Chemother. 2020;64:e01124-20. doi: 10.1128/AAC.01124-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liston S.D., Whitesell L., McLellan C.A., Mazitschek R., Petraitis V., Petraitiene R., Kavaliauskas P., Walsh T.J., Cowen L.E. Antifungal Activity of Gepinacin Scaffold Glycosylphosphatidylinositol Anchor Biosynthesis Inhibitors with Improved Metabolic Stability. Antimicrob. Agents Chemother. 2020;64:e00899-20. doi: 10.1128/AAC.00899-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohammad H., Eldesouky H.E., Hazbun T., Mayhoub A.S., Seleem M.N. Identification of a Phenylthiazole Small Molecule with Dual Antifungal and Antibiofilm Activity Against Candida albicans and Candida auris. Sci. Rep. 2019;9:18941. doi: 10.1038/s41598-019-55379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiederhold N.P., Shawn R.L., Najvar L.K., Berkow E.L., Jaramillo R., Olivo M., Garvey E.P., Yates C.M., Schotzinger R.J., Catano G., et al. The Fungal Cyp51-Specific Inhibitor VT-1598 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob. Agents Chemother. 2019;63:e02233-18. doi: 10.1128/AAC.02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Break T.J., Desai J.V., Healey K.R., Natarajan M., Ferre E.M.N., Henderson C., Zelazny A., Siebenlist U., Yates C.M., Cohen O.J., et al. VT-1598 inhibits the in vitro growth of mucosal Candida strains and protects against fluconazole-susceptible and -resistant oral candidiasis in IL-17 signalling-deficient mice. J. Antimicrob. Chemother. 2018;73:2089–2094. doi: 10.1093/jac/dky170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiederhold N.P., Patterson H.P., Tran B.H., Yates C.M., Schotzinger R.J., Garvey E.P. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species endemic fungi including Coccidioides species Aspergillus species and Rhizopus arrhizus. J. Antimicrob. Chemother. 2018;73:404–408. doi: 10.1093/jac/dkx410. [DOI] [PubMed] [Google Scholar]
- 115.Garvey E.P., Sharp A.D., Warn P.A., Yates C.M., Schotzinger R.J. The novel fungal CYP51 inhibitor VT-1598 is efficacious alone and in combination with liposomal amphotericin B in a murine model of cryptococcal meningitis. J. Antimicrob. Chemother. 2018;73:2815–2822. doi: 10.1093/jac/dky242. [DOI] [PubMed] [Google Scholar]
- 116.Wiederhold N.P., Shubitz L.F., Najvar L.K., Jaramillo R., Olivo M., Catano G., Trinh H.T., Yates C.M., Schotzinger R.J., Garvey E.P., et al. The novel fungal Cyp51 inhibitor VT-1598 is efficacious in experimental models of central nervous system coccidioidomycosis caused by Coccidioides posadasii and Coccidioides immitis. Antimicrob. Agents Chemother. 2018;62:e02258-17. doi: 10.1128/AAC.02258-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rudramurthy S.M., Colley T., Abdolrasouli A., Ashman J., Dhaliwal M., Kaur H., Armstrong-James D., Strong P., Rapeport G., Schelenz S., et al. In vitro antifungal activity of a novel topical triazole PC945 against emerging yeast Candida auris. J. Antimicrob. Chemother. 2019;74:2943–2949. doi: 10.1093/jac/dkz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bartizal K., Daruwala P., Locke J.B., Ong V., Sandison T., Thye D. Dosing regimens of CD101 acetate for the treatment of fungal infections. WO 2017161016 A1 20170921. PCT Int. Appl. (2017) 2017 Sep 21;
- 119.Bartizal K., Daruwala P., Ong V. Methods for treating fungal infections by administering to the subject an antifungal compound CD101. WO 2018191692 A1 20181018. PCT Int. Appl. (2018) 2018 Oct 18;
- 120.Berkow E.L., Lockhart S.R. Activity of CD101 a long-acting echinocandin against clinical isolates of Candida auris. Diagn. Microbiol. Infect. Dis. 2018;90:196–197. doi: 10.1016/j.diagmicrobio.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 121.Hager C.L., Larkin E.L., Long L.A., Ghannoum M.A. Evaluation of the efficacy of rezafungin a novel echinocandin in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J. Antimicrob. Chemother. 2018;73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lepak A.J., Zhao M., Andes D.R. Pharmacodynamic Evaluation of Rezafungin (CD101) against Candida auris in the Neutropenic Mouse Invasive Candidiasis Model. Antimicrob. Agents Chemother. 2018;62:e01572-18. doi: 10.1128/AAC.01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tóth Z., Forgács L., Locke J.B., Kardos G., Nagy F., Kovács R., Szekely A., Borman A.M., Majoros L. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2019;74:3505–3510. doi: 10.1093/jac/dkz390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Helleberg M., Jørgensen K.M., Krøger Hare R., Datcu R., Chowdhary A., Arendrup M.A. Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 2020;64:e02438-19. doi: 10.1128/AAC.02438-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. [(accessed on 7 December 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03667690?term=rezafungin&draw=2&rank=2.
- 126.Van Arnam E., Sit C.S.W., Ruzzini A.C., Clardy J.C., Currie C., Pinto-Tomas A.A. Antifungal compounds comprising selvamicin and anologs. WO 2017210565 A1 20171207. PCT Int. Appl. (2017) 2017 Dec 7;
- 127.Arias L.S., Butcher M.C., Short B., McKloud E., Delaney C., Kean R., Monteiro D.R., Williams C., Ramage G., Brown J.L. Chitosan Ameliorates Candida auris Virulence in a Galleria mellonella Infection Model. Antimicrob. Agents Chemother. 2020;64:e00476-20. doi: 10.1128/AAC.00476-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chauhan R., Loonker S. Synthesis characterization and biological evaluation of chitosan epoxy n-methyl piperazine as antimicrobial agent. Int. J. Pharm. Sci. Rev. Res. 2017;45:266–270. [Google Scholar]
- 129.Dekkerová J., Lopez-Ribot J.L., Bujdáková H. Activity of anti-CR3-RP polyclonal antibody against biofilms formed by Candida auris a multidrug-resistant emerging fungal pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:101–108. doi: 10.1007/s10096-018-3400-x. [DOI] [PubMed] [Google Scholar]
- 130.Srivastava V., Ahmad A. Abrogation of pathogenic attributes in drug resistant Candida auris strains by farnesol. PLoS ONE. 2020;15:0233102. doi: 10.1371/journal.pone.0233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagy F., Vitalis E., Jakab A., Borman A.M., Forgacs L., Toth Z., Majoros L., Kovacs R. In vitro and in vivo Effect of Exogenous Farnesol Exposure Against Candida auris. Front. Microbiol. 2020;11:957. doi: 10.3389/fmicb.2020.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nagy F., Toth Z., Daroczi L., Szekely A., Borman A.M., Majoros L., Kovacs R. Farnesol increases the activity of echinocandins against Candida auris biofilms. Med. Mycol. 2020;58:404–407. doi: 10.1093/mmy/myz057. [DOI] [PubMed] [Google Scholar]
- 133.Kim H.-R., Eom Y.-B. Antifungal and anti-biofilm effects of 6-shogaol against Candida auris. J. Appl. Microbiol. 2020 doi: 10.1111/jam.14870. [DOI] [PubMed] [Google Scholar]
- 134.Edouarzin E., Horn C., Paudyal A., Zhang C., Lu J., Tong Z., Giaever G., Nislow C., Veerapandian R., Hua D.H., et al. Broad-spectrum antifungal activities and mechanism of drimane sesquiterpenoids. Microb. Cell. 2020;7:146–159. doi: 10.15698/mic2020.06.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Juanjuan L., Qianqian L., Changzhong W., Jing S., Tianming W., Daqiang W., Kelong M., Guiming Y., Dengke Y. Antifungal evaluation of traditional herbal monomers and their potential for inducing cell wall remodeling in Candida albicans and Candida auris. Biofouling. 2020;36:319–331. doi: 10.1080/08927014.2020.1759559. [DOI] [PubMed] [Google Scholar]
- 136.Tran H.N.H., Graham L., Adukwu E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020;104:8911–8924. doi: 10.1007/s00253-020-10829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hasaballah A.I., El-Naggar H.A. Antimicrobial activities of some marine sponges and its biological repellent effects against Culex pipiens (Diptera: Culicidae) Ann. Res. Rev. Biol. 2017;12:e1007460. doi: 10.9734/ARRB/2017/32450. [DOI] [Google Scholar]
- 138.Zhang F., Zhao M., Braun D.R., Ericksen S.S., Piotrowski J.S., Nelson J., Peng J., Ananiev G.E., Chanana S., Barns K., et al. A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science. 2020;370:974–978. doi: 10.1126/science.abd6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao M., Zhang F., Zarnowski R., Barns K.J., Jones R., Fossen J.L., Sanchez H., Rajski S.R., Audhya A., Bugni T.S., et al. Turbinmicin inhibits Candida biofilm growth by disrupting fungal vesicle-mediated trafficking. J. Clin. Investig. 2021;131:e145123. doi: 10.1172/JCI145123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bugni T.S., Zhang F., Braun D.R., Andes D.R., Zhao M. Turbinmicin compounds compositions and uses thereof. WO 2020146155 A1 20200716. PCT Int. Appl. (2020) 2020 Jul 16;
- 141.López-Abarrategui C., McBeth C., Mandal S.M., Sun Z.J., Heffron G., Alba-Menéndez A., Migliolo L., Reyes-Acosta O., García-Villarino M., Nolasco D.O., et al. Cm-p5: An antifungal hydrophilic peptide derived from the coastal mollusk Cenchritis muricatus (Gastropoda: Littorinidae) FASEB J. 2015;29:3315–3325. doi: 10.1096/fj.14-269860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morales-Vicente F.E., Gonzalez-Garcia M., Diaz Pico E., Moreno-Castillo E., Garay H.E., Rosi P.E., Jimenez A.M., Campos-Delgado J.A., Rivera D.G., Chinea C., et al. Design of a Helical-Stabilized Cyclic and Nontoxic Analogue of the Peptide Cm-p5 with Improved Antifungal Activity. ACS Omega. 2019;4:19081–19095. doi: 10.1021/acsomega.9b02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kubiczek D., Raber H., Rosenau F., Gonzalez-Garcia M., Otero-Gonzalez A.J., Morales-Vicente F., Staendker L. Derivates of the Antifungal Peptide Cm-p5 Inhibit Development of Candida auris Biofilms In Vitro. Antibiotics. 2020;9:363. doi: 10.3390/antibiotics9070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramachandran R., Shrivastava M., Narayanan N.N., Thakur R.L., Chakrabarti A., Roy U. Evaluation of Antifungal Efficacy of Three New Cyclic Lipopeptides of the Class Bacillomycin from Bacillus subtilis RLID 12.1. Antimicrob. Agents Chemother. 2017;62:e01457-17. doi: 10.1128/AAC.01457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silva P.M., Goncalves S., Santos N.C. Defensins: Antifungal lessons from eukaryotes. Front. Microbiol. 2014;5:97. doi: 10.3389/fmicb.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Swidergall M., Ernst J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell. 2014;13:950–957. doi: 10.1128/EC.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duncan V.M.S., O’Neil D.A. Commercialization of antifungal peptides. Fungal Biol. Rev. 2013;26:156–165. doi: 10.1016/j.fbr.2012.11.001. [DOI] [Google Scholar]
- 148.Vriens K., Cammue B.P.A., Thevissen K. Antifungal plant defensins: Mechanisms of action and production. Molecules. 2014;19:12280–12303. doi: 10.3390/molecules190812280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Batoni G., Maisetta G., Brancatisano F.L., Esin S., Campa M. Use of antimicrobial peptides against microbial biofilms: Advantages and limits. Curr. Med. Chem. 2011;18:256–279. doi: 10.2174/092986711794088399. [DOI] [PubMed] [Google Scholar]
- 150.Pathirana R.U., Friedman J., Norris H.L., Salvatori O., McCall A.D., Kay J., Edgerton M. Fluconazole-Resistant Candida auris Is Susceptible to Salivary Histatin 5 Killing and to Intrinsic Host Defenses. Antimicrob. Agents Chemother. 2018;62:e01872-17. doi: 10.1128/AAC.01872-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Basso V., Garcia A., Tran D.Q., Schaal J.B., Tran P., Ngole D., Aqeel Y., Tongaonkar P., Ouellette A.J., Selsted M.E. Fungicidal Potency and Mechanisms of θ-Defensins against Multidrug-Resistant Candida Species. Antimicrob. Agents Chemother. 2018;62:e00111-18. doi: 10.1128/AAC.00111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dal Mas C., Rossato L., Shimizu T., Oliveira E.B., da Silva Junior P.I., Meis J.F., Colombo A.L., Hayashi M. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida auris an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules. 2019;9:205. doi: 10.3390/biom9060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Eijk M., Boerefijn S., Cen L., Rosa M., Morren M.J.H., van der Ent C.K., Kraak B., Dijksterhuis J., Valdes I.D., Haagsman H.P., et al. Cathelicidin-inspired antimicrobial peptides as novel antifungal compounds. Med. Mycol. 2020;58:1073–1084. doi: 10.1093/mmy/myaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hashemi M.M., Rovig J., Holden B.S., Taylor M.F., Weber S., Wilson J., Hilton B., Zaugg A.L., Ellis S.W., Yost C.D., et al. Ceragenins are active against drug-resistant Candida auris clinical isolates in planktonic and biofilm forms. J. Antimicrob. Chemother. 2018;73:1537–1545. doi: 10.1093/jac/dky085. [DOI] [PubMed] [Google Scholar]
- 155.Karlsson M., Atanasova L., Jensen D.F., Zeilinger S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0016-2016. [DOI] [PubMed] [Google Scholar]
- 156.Junker K., Bravo Ruiz G., Lorenz A., Walker L., Gow N., Wendland J. The mycoparasitic yeast Saccharomycopsis schoenii predates and kills multi-drug resistant Candida auris. Sci. Rep. 2018;8:14959. doi: 10.1038/s41598-018-33199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bastos R.W., Rossato L., Valero C., Lagrou K., Colombo A.L., Goldman G.H. Potential of Gallium as an Antifungal Agent. Front. Cell. Infecti. Microbiol. 2019;9:414. doi: 10.3389/fcimb.2019.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dennis E.K., Kim J.H., Parkin S., Awuah S.G., Garneau-Tsodikova S. Distorted Gold(I)-Phosphine Complexes as Antifungal Agents. J. Med. Chem. 2020;63:2455–2469. doi: 10.1021/acs.jmedchem.9b01436. [DOI] [PubMed] [Google Scholar]
- 159.Kosgey J.C., Jia L., Fang Y., Yang J., Gao L., Wang J., Nyamao R., Cheteu M., Tong D., Wekesa V., et al. Probiotics as antifungal agents: Experimental confirmation and future prospects. J. Microbiol. Meth. 2019;162:28–37. doi: 10.1016/j.mimet.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 160.Kunyeit L., Kurrey N.K., Anu-Appaiah K.A., Rao R.P. Probiotic Yeasts Inhibit Virulence of Non-albicans Candida Species. mBio. 2019;10:e02307-19. doi: 10.1128/mBio.02307-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.York A. A probiotic for candidiasis? Nat. Rev. Microbiol. 2019;17:723. doi: 10.1038/s41579-019-0296-0. [DOI] [PubMed] [Google Scholar]
- 162.Rossoni R.D., de Barros P.P., Mendonça I.D.C., Medina R.P., Silva D., Fuchs B.B., Junqueira J.C., Mylonakis E. The Postbiotic Activity of Lactobacillus paracasei 28.4 Against Candida auris. Front. Cell. Infect. Microbiol. 2020;10:397. doi: 10.3389/fcimb.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sherin P., Kuriakose S. Synthesis of Superparamagnetic Iron Oxide Nanoparticles Stabilized by Biocompatible Supramolecular β-Cyclodextrin for Biomedical Applications. Mater. Today Proc. 2019;11:1030–1035. doi: 10.1016/j.matpr.2018.12.034. [DOI] [Google Scholar]
- 164.Gangadoo S., Elbourne A., Medvedev A.E., Cozzolino D., Truong Y.B., Crawford R.J., Wang P.Y., Truong V.K., Chapman J. Facile Route of Fabricating Long-Term Microbicidal Silver Nanoparticle Clusters against Shiga Toxin-Producing Escherichia coli O157:H7 and Candida auris. Coatings. 2020;10:28. doi: 10.3390/coatings10010028. [DOI] [Google Scholar]
- 165.Lara H.H., Ixtepan-Turrent L., Jose Yacaman M., Lopez-Ribot J. Inhibition of Candida auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl. Mater. Interfaces. 2020;12:21183–21191. doi: 10.1021/acsami.9b20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cleare L.G., Li K.L., Abuzeid W.M., Nacharaju P., Friedman J.M., Nosanchuk J.D. NO Candida auris: Nitric Oxide in Nanotherapeutics to Combat Emerging Fungal Pathogen Candida auris. J. Fungi. 2020;6:85. doi: 10.3390/jof6020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vazquez-Munoz R., Lopez F.D., Lopez-Ribot J.L. Bismuth Nanoantibiotics Display Anticandidal Activity and Disrupt the Biofilm and Cell Morphology of the Emergent Pathogenic Yeast Candida auris. Antibiotics. 2020;9:461. doi: 10.3390/antibiotics9080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shukla A., Vera-Gonzalez N. Aspartic protease-triggered antifungal hydrogel. US 20190151455 A1 20190523. U.S. Pat. Appl. Publ. (2019) 2019 May 23;
- 169.Kubiczek D., Flaig C., Raber H., Dietz S., Kissmann A.K., Heerde T., Bodenberger N., Wittgens A., González-Garcia M., Kang F., et al. A Cerberus-Inspired Anti-Infective Multicomponent Gatekeeper Hydrogel against Infections with the Emerging “Superbug” Yeast Candida auris. Macromol. Biosci. 2020;20:2000005. doi: 10.1002/mabi.202000005. [DOI] [PubMed] [Google Scholar]
- 170.Gupta A., Briffa S.M., Swingler S., Gibson H., Kannappan V., Adamus G., Kowalczuk M., Martin C., Radecka I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules. 2020;21:1802–1811. doi: 10.1021/acs.biomac.9b01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 172.Cadnum J.L., Shaikh A.A., Piedrahita C.T., Sankar T., Jencson A.L., Larkin E.L., Ghannoum M.A., Donskey C.J. Effectiveness of disinfectants against Candida auris and other Candida species. Infect. Control. Hosp. Epidemiol. 2017;38:1240–1243. doi: 10.1017/ice.2017.162. [DOI] [PubMed] [Google Scholar]
- 173.Vallabhaneni S., Kallen A., Tsay S., Chow N., Welsh R., Kerins J., Kemble S.K., Pacilli M., Black S.R., Landon E., et al. Investigation of the first seven reported cases of Candida auris a globally emerging invasive multidrug-resistant fungus-United States May 2013-August 2016. Am. J. Transplant. 2017;17:296–299. doi: 10.1111/ajt.14121. [DOI] [PubMed] [Google Scholar]
- 174.Cadnum J.L., Shaikh A.A., Piedrahita C.T., Jencson A.L., Larkin E.L., Ghannoum M.A., Donskey C.J. Relative Resistance of the Emerging Fungal Pathogen Candida auris and Other Candida Species to Killing by Ultraviolet Light. Infect. Control. Hosp. Epidemiol. 2018;39:94–96. doi: 10.1017/ice.2017.239. [DOI] [PubMed] [Google Scholar]
- 175.Maslo C., du Plooy M., Coetzee J. The efficacy of pulsed-xenon ultraviolet light technology on Candida auris. BMC Infect. Dis. 2019;19:540. doi: 10.1186/s12879-019-4137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.de Groot T., Chowdhary A., Meis J.F., Voss A. Killing of Candida auris by UV-C: Importance of exposure time and distance. Mycoses. 2019;62:408–412. doi: 10.1111/myc.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lemons A.R., McClelland T.L., Martin S.B., Lindsley W.G., Green B.J. Inactivation of the multi-drug resistant pathogen Candida auris using ultraviolet germicidal irradiation (UVGI) J. Hosp. Infect. 2020;105:495–501. doi: 10.1016/j.jhin.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.