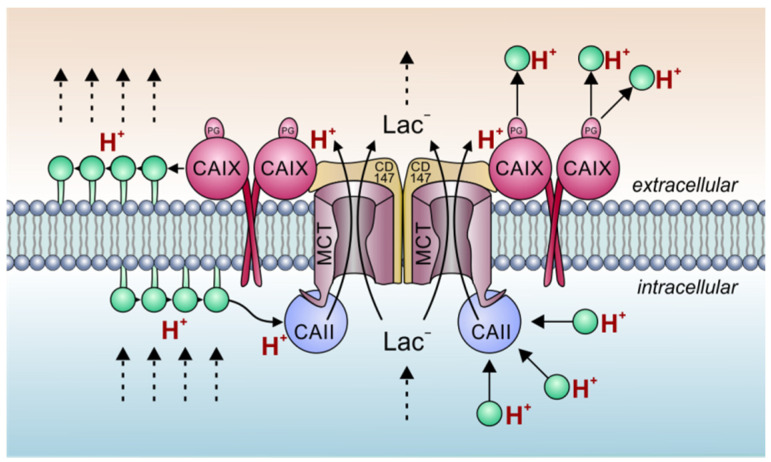
Figure 2.
Carbonic anhydrases function as proton antennae for MCTs. Intracellular and extracellular carbonic anhydrases form a non-catalytic transport metabolon with MCT1 and MCT4. The interaction is independent of CA catalytic activity, but requires a special set of proton-collecting residues in the CA protein (CAII-Glu69/Asp72 and the CAIX-PG domain). Extracellular-facing CAIX binds to the Ig1 domain of the MCT1/4 chaperon CD147, while intracellular CAII binds to the transporter’s C-terminal tail. This binding positions the enzymes close enough to the transporter pore to establish an efficient proton shuttle between transporter and enzymes. During proton/lactate efflux, CAII collects H+ from surrounding protonatable residues of yet unknown identity (green circles) near or at the plasma membrane and shuttles them to the transporter. On the extracellular site, CAIX removes H+ from the transporter pore and shuttles them to protonatable residues at the extracellular face of the plasma membrane or in the extracellular space. This rapid exchange of H+ impairs the formation of proton microdomains around the transporter pore and drives the efflux of protons and lactate out of the cell. Note that both CAIX and the MCT1/4-CD147 complex exist as dimers at the cell membrane [137,138]. In the figure, dotted arrows symbolize ion diffusion. Solid arrows symbolize ion transport or proton transfer.