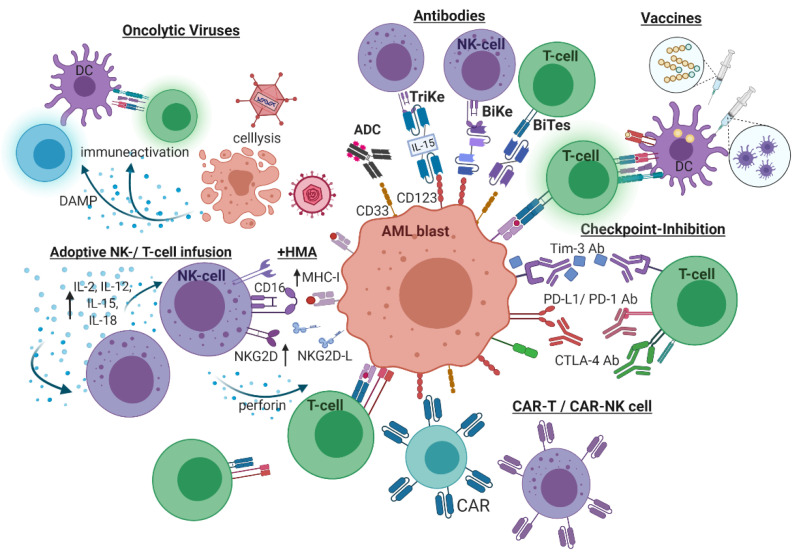
Figure 2.
Immunotherapeutic approaches in AML. Checkpoint blockade prevents immune-suppressive signaling through programmed cell-death protein-1/programmed cell-death ligand (PD-1/PD-L1), cytotoxic T-lymphocyte associated protein 4 (CTLA-4), T-cells immunoglobulin-mucin 3 (Tim-3)/galactin-9 (Gal-9), as prominent examples. Cellular-based immunotherapeutic approaches comprise adoptive T- and NK cell infusion. Exposure to activating cytokines (IL-2, -12, -15, and -18) augments NK-mediated cytotoxicity. Additional administration of hypomethylating agents (HMA) potentiate cellular immune response. Chimeric antigen receptor (CAR) is another strategy to improve leukemia directed T- and NK cell reactivity. Leukemia-associated antigen-directed antibodies stimulate antibody-dependent cell-mediated cytotoxicity (ADCC). Antibody–drug conjugates (ADC) are linked to cytotoxic agents to directly lyse targeted leukemic blasts. Bispecific T-cell engager (BiTE), bi- and tri-specific NK cell engager (BiKE, TriKE) bind and crosslink leukemic antigens to T- and NK cells facilitating anti-leukemic reactivity. Vaccine therapy can be either based on antigens or dendritic cells, presenting neoantigens to T-cells resulting in leukemia-directed cytotoxicity. Oncolytic viruses directly lyse infected AML cells and specifically reinforce anti-leukemic immunogenicity, i.e., through released specific damage-associated molecular patterns (DAMP). Figure 2 was created with with biorender.com (accessed on 20 February 2021).