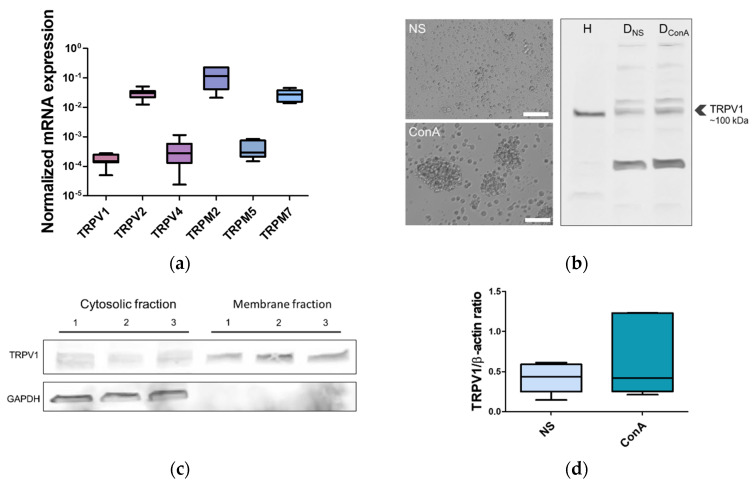
Figure 3.
Expression of TRP ion channels in the canine PBMC, with special emphasis given to TRPV1. (a) mRNA expression of selected TRP ion channels in the canine PBMC. TRPV2, TRPM2, and TRPM7 were found to exhibit high expression, while TRPV1, TRPV4, and TRPM5 were characterized by low expression. Expression level is represented as arbitrary units. Data are shown as a dot plot, with mean ± s.e.m. (n = 7 animals). Different letters indicate significant differences (one-way ANOVA, followed by Tukey’s post hoc, p < 0.0001). (b) TRPV1 protein level in the canine PBMC. TRPV1 antibody recognized a major band around ~100 kDa in the lysate of the canine PBMC. As a control of antibody specificity, we used PBMC lysate from a human (H). Western blot was run on PBMC that were not stimulated (DNS) or activated with plant mitogen concanavalin-A (DConA) for 24 h. Activation was confirmed by microscopy (activated cells groups into clusters). Scale bars, 50 µm. (c) Western blot’s densitometric quantification. We did not found significant differences between NS and ConA treated groups. Β-actin was used as a loading control. Values are means ± s.e.m., n = 5, Student’s t-test, p < 0.05. (d) Western blot of cytosolic and membranous fractions from canine PBMC. TRPV1 localizes in plasma membrane; however, immunoreactive bands in cytosolic fractions suggest that it might also localize in other subcellular compartments. As a control, we used GAPDH (~40 kDa). Immunoreactive bands for GAPDH were found only in the cytosolic fractions (n = 7).