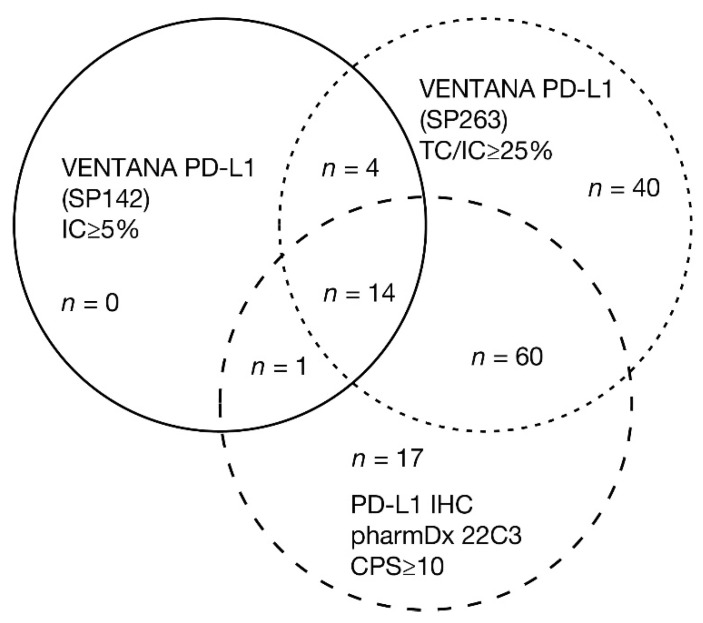
Figure 3.
UC specimens classified as PD-L1 high using commercially available PD-L1 assays. The figure shows the overlap of positive cases using three commercially available PD-L1 assays. In an assay comparison study, 335 UC tumor samples were stained and scored with the algorithm and cutpoint associated with that assay [48]. Of the 40% of samples deemed PD-L1 high using any assay, 12% were uniquely identified by the VENTANA PD-L1 (SP263) Assay, 5% by the PD-L1 IHC pharmDx 22C3 Assay, and none were unique to the VENTANA PD-L1 (SP142) Assay. The highest overlap in assays was observed between the VENTANA PD-L1 (SP263) Assay and PD-L1 IHC pharmDx 22C3 Assay, where 22% of samples were PD-L1 high with either assay. If extrapolated to a clinical setting, this exploratory study indicates that of 100 patients tested with all three assays, only four patients would be deemed PD-L1 high with all three assays (with acknowledgment to Marietta Scott, Precision Medicine, AstraZeneca, Cambridge, UK, for analysis of the data).