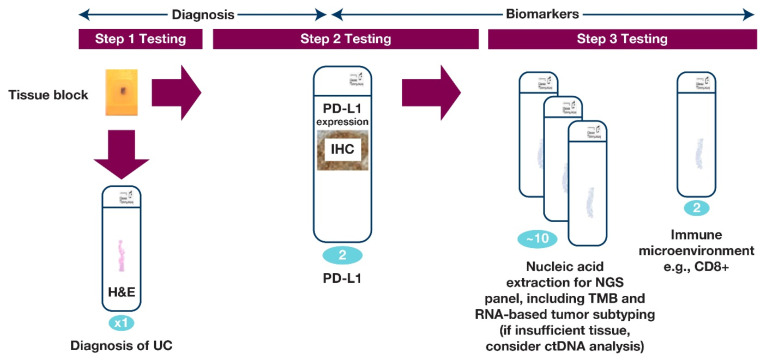
Figure 4.
UC biomarkers workflow, which is an integrated platform to report PD-L1 and other biomarkers. Figure 4 illustrates a prospective integrated protocol for multiple biomarker testings on samples from patients with UC. The numbers of sections required are shown in blue. Ideally, in the future, step 1 testing would be combined with step 2 testing to accelerate information to the clinician and inform potential treatment. A minimum of two slides are required for PD-L1 testing and tissue requirements for next-generation sequencing (NGS) may vary according to the testing platform used.