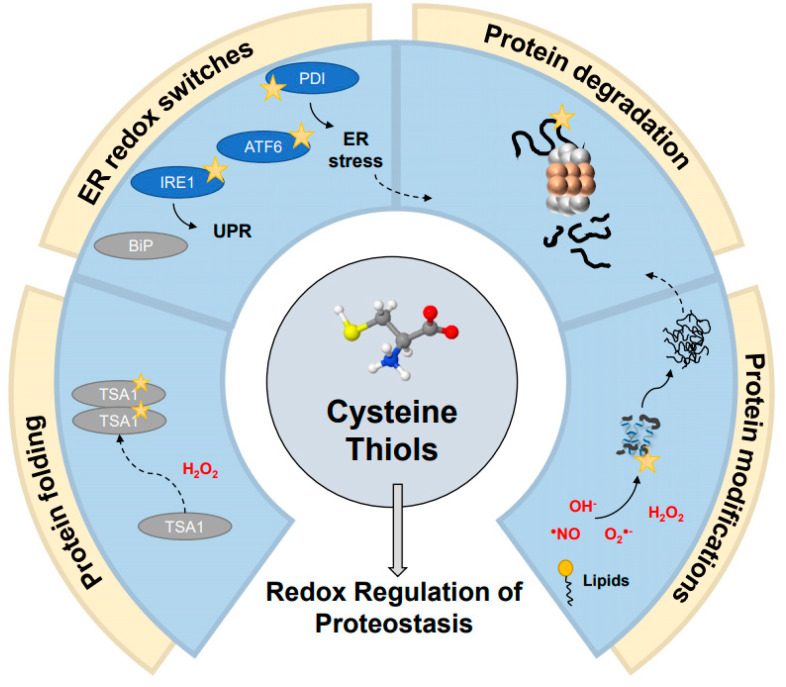
Figure 2.
Redox switches in proteostasis. Cysteine thiol switches are involved in regulating various aspects of proteostasis, such as protein folding, ER quality control and the unfolded protein response (UPR), protein degradation across various stages, and protein modifications and maturation. Numerous examples of redox-sensitive thiols have been found across each of these stages of protein quality control, such as redox regulation of the peroxiredoxin TSA1, the ER protein disulfide isomerase (PDI) and BiP chaperones, members of the UPR mechanism IRE1 and ATF6, and the proteasome itself. These emerge from oxidative modifications of varying sorts, including reactive oxygen species (ROS), reactive nitrogen species (RNS), and lipid modifications.