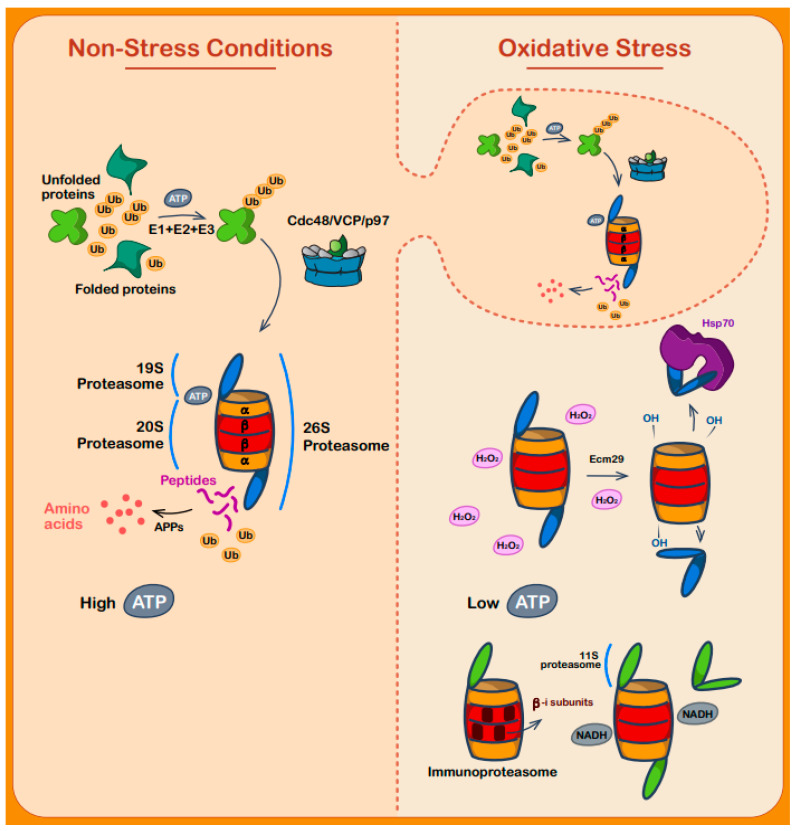
Figure 4.
Proteasome-mediated protein degradation during oxidative stress. To the left, under non-stress conditions and in a high ATP environment, unfolded and misfolded proteins undergo degradation by the ubiquitin system by tagging the targeted proteins with ubiquitin (as carried out by the E1, E2, and E3 enzymes), then reaching the 26S proteasome with the aid of different shuttle proteins. The client proteins are initially recognized by the 19S particle. Following binding and utilization of the energy stored in ATP molecules, the substrate unfolds to its primary structure and enters the hollow barrel-like structure of the 20S particle. The substrate is then degraded into peptides by the catalytic units of the beta-subunits, while aminopeptidases (APPs) break them down to amino acids after the peptides exit the proteasome. To the right, oxidative stress conditions alter the cell mechanism of dealing with misfolded and unfolded proteins. The previously described ubiquitin-based degradation system is minimized by the decrease in ATP molecules, and the 26S proteasome comes apart. The 19S and 20S particles split, mediated by the Ecm29 protein and Hsp70. While the 19S is held by Hsp70, the now-oxidized 20S particle begins to function by itself in an ATP-independent manner and degrades unfolded and misfolded proteins. Under these conditions, another kind of proteasome—the 20Si (immunoproteasome)—is upregulated. This proteasome is combined with 3 different beta-subunits (see text), enhancing the proteasome’s catalytic abilities. The 11S or PA28 subunits are upregulated, serving as an alternative regulatory unit for the 20S and 20Si proteasomes, while NADH stabilizes the proteasome structure in the absence of ATP.