Figure 3.
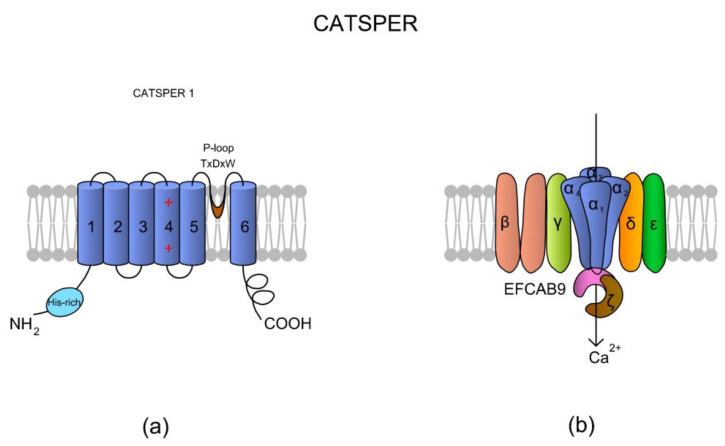
A topological and spatial structure of CatSper. (a) The α1 subunit created by CatSper1. Like most voltage-gated channels, each α subunit contains six transmembrane domains (TM1–TM6) creating two physiologically distinctive regions, namely the voltage-sensing domain (VSD; TM1–4) and pore-forming region (TM5–6). Each TM4 contains several (two to six) positively charged amino acid residues that serve as voltage sensors (reviewed in Reference [57]). Voltage slopes move TM4, resulting in conformational changes that open and close the channel pore [64]. Additionally, a short and hydrophobic cyclic structure linking TM5–6 contains a conserved homologous amino acid sequence (T × D × W), which selectively permits Ca2+ influx. The N-terminus of CatSper 1 contains a specific histidine-rich region that might be involved in the pH regulation of CatSper activity. (b) The topological localizations of all auxiliary subunits are not randomly organized. The auxiliary CatSperβ subunit has two predicted TMs that are separated by a large (ca. 1000 amino acids) extracellular loop [64], whereas CatSperγ, CatSperδ, and CatSperε feature only one TM. Brown et al. [69] suggested that CatSperζ is a late evolutionary adaptation to maximize fertilization success inside the female mammalian reproductive tract. The predicted topology of Hwang et al. [62] situates the CatSperζ and EFCAB9 subunits as a cytoplasm complex that is located just below the CatSper 1–4 subunits. This complex interacts with the channel pore as a gatekeeper. The increase in pHi causes Ca2+ binding to highly conserved EF-hands of EFCAB9, leading to dissociation of the EFCAB9-CatSperζ complex and full activation of the channel. Accordingly, EFCAB9-CatSperζ appears to be responsible for both modulation of the channel activity and organization of the CatSper domains [62]. The scheme has been prepared based on Reference [62].