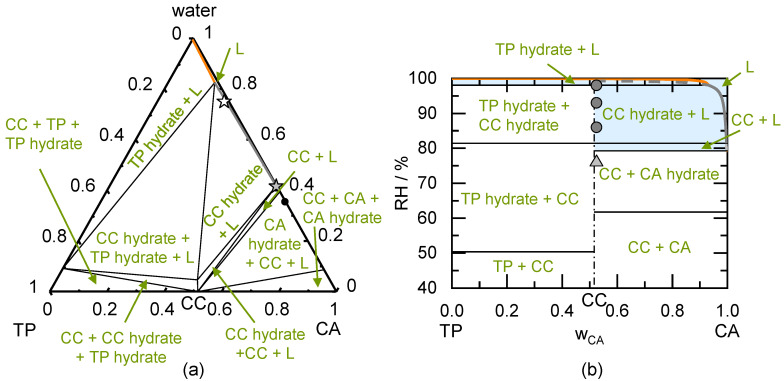
Figure 6.
(a) Modeled ternary phase diagram of theophylline/citric acid in mass fractions at 298.15 K. The TP hydrate solubility line starting from the left axis, the CC hydrate solubility line below is intersecting into the CC solubility line. The CA hydrate solubility line is starting from the right axis (cannot be seen in this diagram). The thin solid lines indicate the phase boundaries. The circle indicates the eutectic point of CA hydrate and the CC anhydrate, the grey star is the eutectic point of the CC hydrate and CC anhydrate, and the white star is the eutectic point of the CC hydrate and TP hydrate obtained from literature [15]; (b) Predicted phase diagram of theophylline/citric acid showing the thermodynamically stable phases as function of the mass fraction in the dry system and RH at 298.15 K. The thick solid TP hydrate deliquescence line starting from the left axis intersecting the thick solid CC hydrate deliquescence line, which is intersecting in the CC anhydrate deliquescence line. The thick solid line of CA hydrate starts from the right axis (cannot be seen in this diagram). The thick dark-grey dashed line indicates the metastable CC hydrate deliquescence line. The thin solid lines indicate the phase boundaries, the thin dash-dotted line indicates the CC stoichiometry. Symbols indicate results of CC hydrate stability measurements after 49 days of storage. The light grey triangle represent transformation to the CC anhydrate. Dark grey circles indicate deliquescence during storage of CC hydrate.