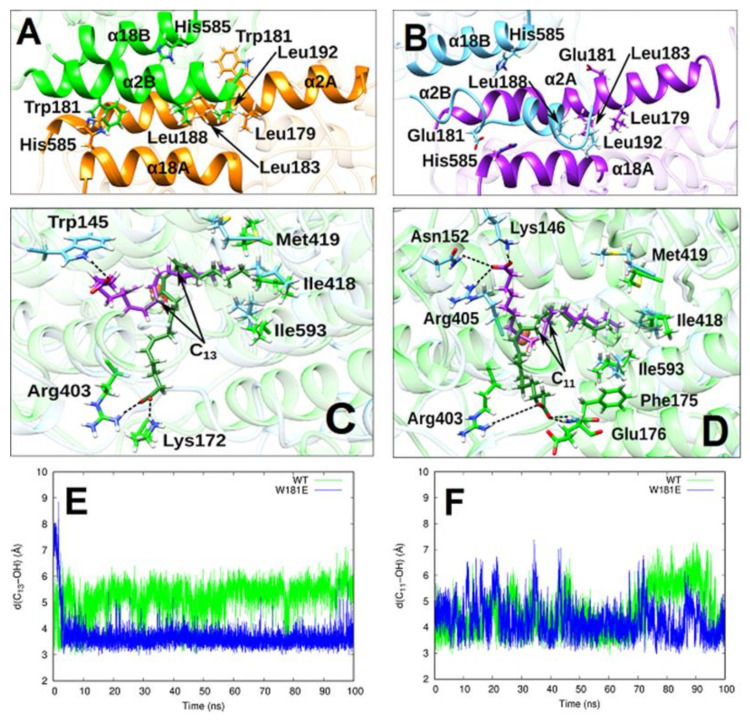
Figure 4.
Structural consequences of Trp181Glu exchange on dimer formation and substrate alignment. (A) Inter-monomer interface of wild-type rabbit ALOX15. Secondary structural elements of conformer A are shown in mustard and those of conformer B are given in green. (B). Inter-monomer interface of the Trp181Glu mutant of rabbit ALOX15. Secondary structural elements of conformer A are shown in light blue and those of conformer B in purple. (C) Most representative binding mode of AA in WT-ALOX15 (dark green) and in Trp181Glu-ALOX15 (purple) dimers. WT-ALOX15 (green with a percentage of transparency) and Trp181Glu-ALOX15 (light blue with a percentage of transparency) backbones have been superimposed. The side-chains of some selected residues for WT-ALOX15 and Trp181Glu-ALOX15 dimers have been displayed in green and light blue, respectively. (D) Most representative binding mode of LA in WT-ALOX15 (dark green) and in Trp181Glu-ALOX15 (purple) dimers. WT-ALOX15 (green with a percentage of transparency) and Trp181Glu-ALOX15 (light blue with a percentage of transparency) backbones have been superimposed. The side-chains of some selected residues for WT-ALOX15 and Trp181Glu-ALOX15 dimers have been displayed in green and light blue, respectively. (E) C13-OH distance in the Trp181Glu-ALOX15-AA (in blue) and in the WT-ALOX15-AA (in green) complexes versus time. (F) C11-OH distance in the Trp181Glu-ALOX15-LA (in blue) and in the WT-ALOX15-LA (in green) complexes versus time.