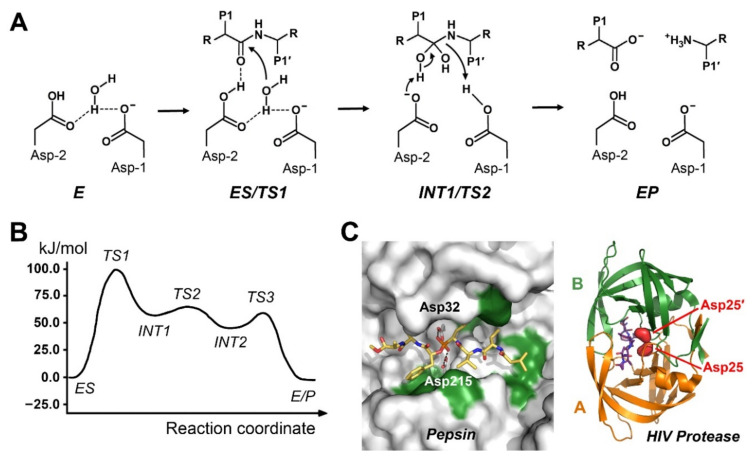
Figure 2.
Mechanism of aspartic proteases. (A) Essentially three major steps takes place, including formation of the Michaelis complex 1. Nucleophilic attack by an activated water molecule with transition state 1 (TS1) and formation of a tetrahedral intermediate (INT). 2. Nitrogen conversion (TS2). 3. Fission of the scissile bond and release of the products with new C- and N-termini (EP). Residue numbering corresponds to pepsin. Two relevant aspartic proteases are shown in Figure 2C. (B) Free energy profile for the pepsin-like protease renin, with an additional reaction step including INT2 and TS3, according to Bras et al. (2012) [33]. (C) Left panel: Active site of pepsin with a phosphonate inhibitor, mimicking TS2 (PDB 1QRP). White areas are polar, green areas are hydrophobic. Right panel: The dimeric HIV protease has one catalytic Asp25 (red spheres) per monomer (PDB 4HVP). A peptidic inhibitor (purple sticks) is bound to the active site as ES analog.