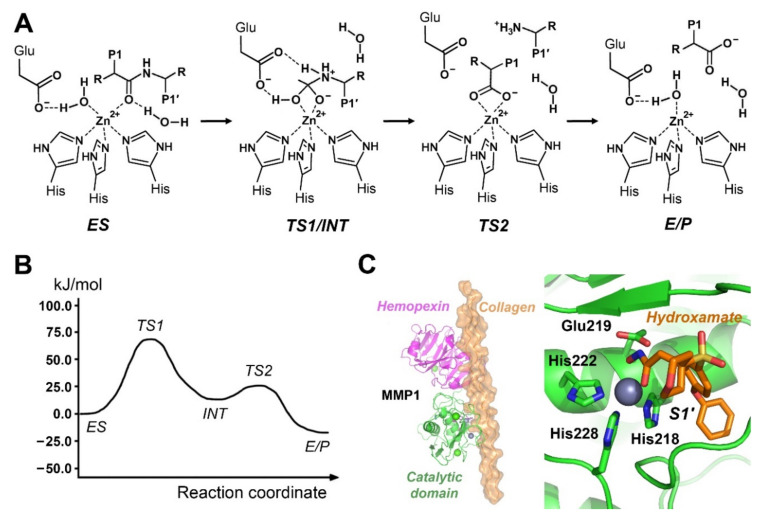
Figure 3.
(A) Mechanism of metalloproteases. 1. Formation of a Michaelis complex (ES) and binding of the carbonyl O of the P1 residue to the catalytic Zn2+, which functions as oxyanion hole. 2. Nucleophilic attack by an activated water molecule, i.e., OH− (TS1), formation of a tetrahedral intermediate and transfer of an H+ to the amide NH of the scissile bond (INT) 3. Cleavage of the scissile bond (TS2) and product release (E/P). Especially step 2 can be further subdivided into more steps. Structural examples with functional relevance are shown in Figure 3C. (B) The relatively simple free energy profile for MMP3 follows Pelmenshikov and Siegbahn (2002) [87]. (C) Left panel: MMP1 in complex with the natural substrate collagen (PDB 966C) corresponds to the ES complex. Right panel: MMP1 active site with a Zn2+ bound hydroxamate inhibitor (PDB 4AUO), which partially resembles the intermediate (INT).