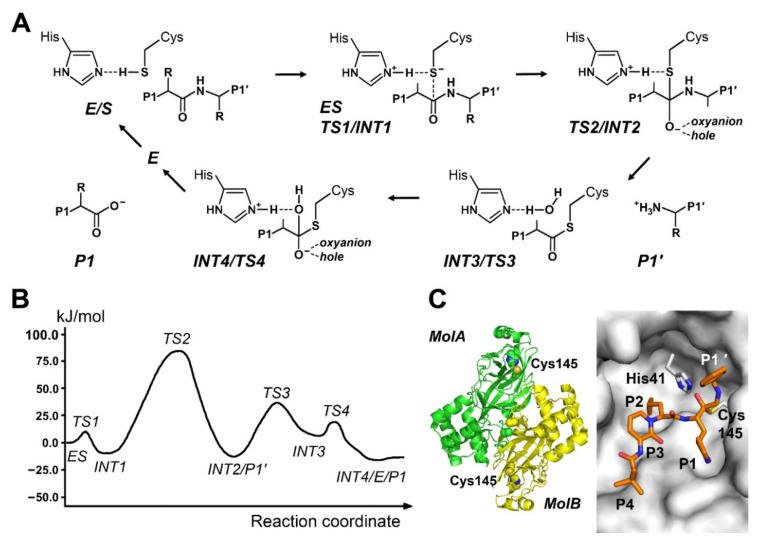
Figure 4.
Mechanism of cysteine proteases. (A) As in the prototypic papain the catalytic residues of the dyad are a Cys and a His. Essentially two major reaction steps take place, namely the acylation and the deacylation, while several sub steps are involved, according to Wei et al. (2013) [99]. 1. Nucleophilic attack by the negatively charged Sγ atom on the carbonyl C of the P1 residue (TS1) and formation of tetrahedral intermediate (INT1). The oxyanion hole stabilizes the negative charge at the carbonyl O atom. 2. Upon protonation of the amide NH group the scissile bond breaks and the P1′ product leaves with a new N-terminus (TS2). 3. The acyl intermediate (INT2) is attacked by the nucleophilic catalytic water (TS3) and forms the second tetrahedral intermediate (INT3). 4. Release of the P1 product with a new carboxy terminus (TS4/INT4 and E/P1). (B) Free energy profile of the above described reaction. (C) The coronavirus SARS-Cov-2 main protease (MPro) is depicted as free enzyme dimer (PDB 6Y2E) on the left and with a covalent ketoamide inhibitor (PDB 6Y2G) as TS2 analog on the right. Some residues of the protease were omitted for clarity.