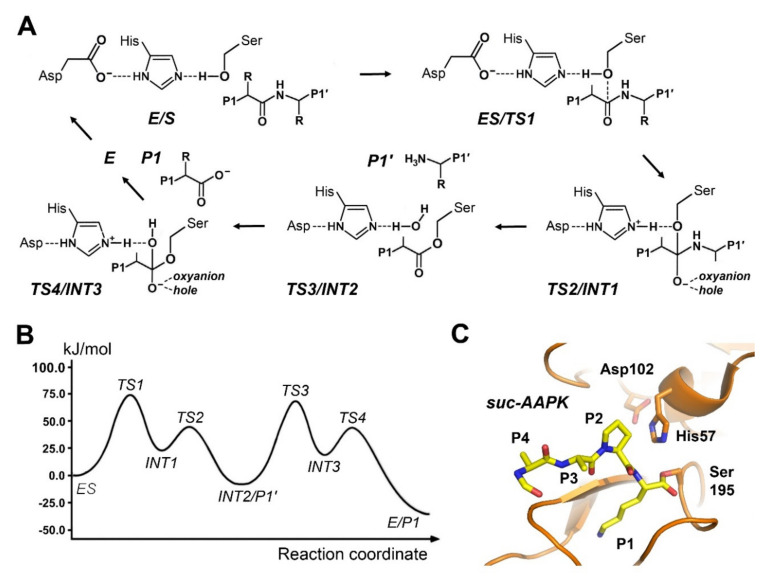
Figure 5.
Mechanism of serine proteases. (A) The basic mechanism resembles the one of cysteine proteases. In a catalytic triad the acidic Asp is required to stabilize the positively charged His, which enhances the nucleophilicity of the Ser Oγ atom. 1. Formation of the Michaelis complex (ES). 2. Nucleophilic attack by the negatively polarized Oγ atom to the carbonyl C of the P1 residue (TS1), resulting in the tetrahedral intermediate with a negative charge at the carbonyl O (INT1), which is bound to oxyanion hole. 3. Protonation of the amide NH group breaks the scissile bond (TS2), with release of the of the P1′ product. 4. The acyl intermediate (INT2) is attacked by the catalytic water (TS3) and forms the second tetrahedral intermediate (INT3). 5. Release of the P1 product (TS4/E/P1) with a new C-terminus. (B) Free energy profile of the reaction in trypsin. (C) Trypsin in complex with a succinyl-Ala-Ala-Pro-Lys inhibitor (PDB 2AGG), which forms a true acyl intermediate, corresponding to INT2.