Figure 1.
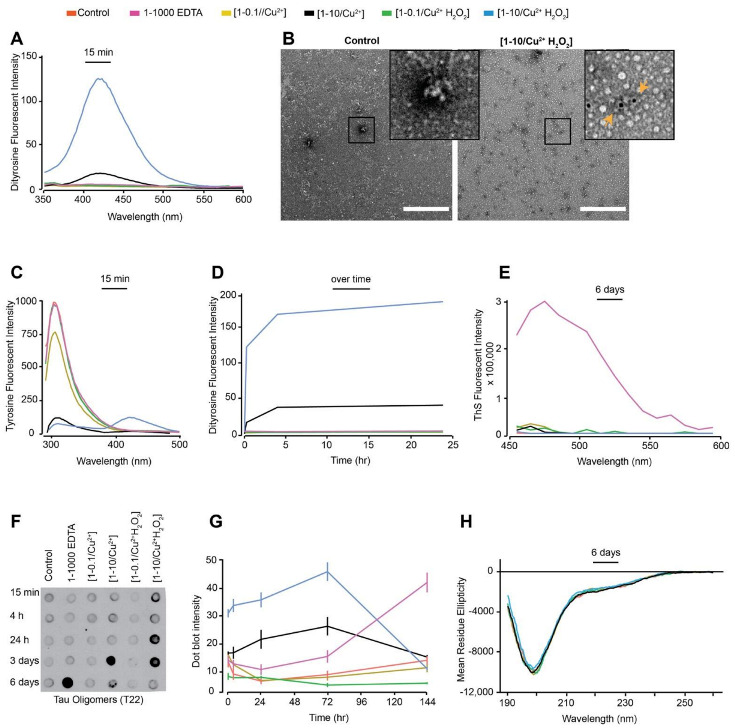
Freshly prepared dGAE (100 μM) was incubated at 37 °C without agitation, alone (control), with 10 μM CuCl2 [1-0.1/Cu2+], 1 mM CuCl2 [1-10/Cu2+], 10 μM CuCl2 in combination with 2.5 mM H2O2 [1-0.1//Cu2+ H2O2], 1 mM CuCl2 in combination with 2.5 mM H2O2 [1-10/Cu2+ H2O2) or with 100 mM EDTA (1-1000 EDTA) (see Table 1 for condition details). DiY signal was collected 15 min post-incubation using fluorescent excitation/emission 320 nm/340–600 nm, with DiY peak signal observed between 400–420 nm (A). The presence of DiY in dGAE was confirmed using TEM immunogold-labelling where black dots indicate DiY detected with mouse anti-DiY antibody (see orange arrows in the insert). Scale bar, 500 nm. (B). Tyrosine signal was collected at 15 min of incubation using an excitation/emission 280 nm/290–500 nm, with peak tyrosine signal observed at 305 nm (C). DiY signal was collected again at 4 h and 24 h to follow DiY formation over 24 h duration (D). ThS fluorescence intensity was conducted after six days incubation to observe the degree of self-assembly using excitation/emission of 440/460–600 nm (E). Immunoblotting using T22 antibody identifies the presence of tau oligomers (F). Quantification of the immunoblot signal suggest a high level of tau oligomers early-on in the [1-0.1//Cu2+ H2O2] and [1-0.1//Cu2+] samples (G). CD analysis at six days post-incubation showed that all the samples had a comparable level of random-coil secondary structure (H).
