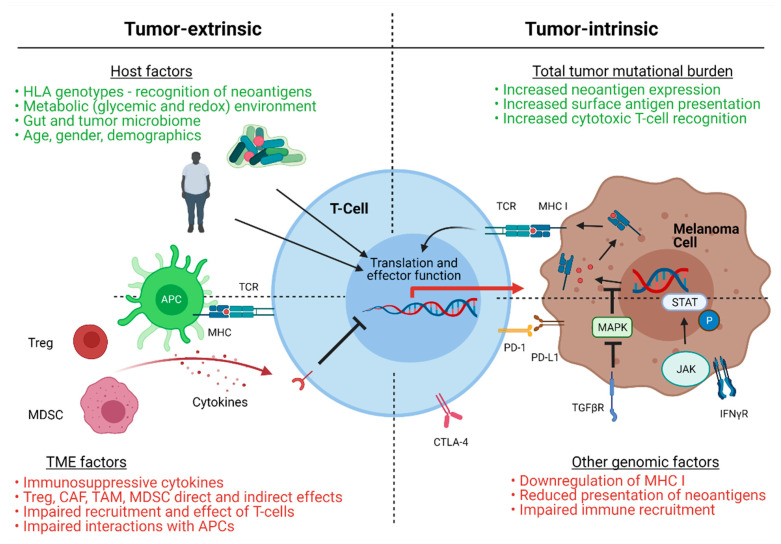
Figure 1.
Proposed mechanisms of resistance to immune checkpoint inhibition in melanoma. In green are factors associated with potential immune checkpoint inhibitor responsiveness, while red highlights factors associated with potential resistance to therapy. Tumor extrinsic mechanisms include host factors and tumor microenvironment (TME) factors. Host factors include: immune recognition via specific human leukocyte antigen (HLA) genotypes, metabolic factors, such as obesity and diabetes; the gut/tumor microbiome; demographics, such as age or gender. TME factors include: an immunosuppressive cytokine milieu; the presence of regulatory T-cells (Treg); cancer associated fibroblasts (CAF); tumor associated macrophages (TAM)); myeloid derived suppressor cells (MDSC) and other cell types. Each elicits specific effects on T-cell recruitment and activity, and may impair interactions between antigen presenting cells (APCs) and T-cells. Tumor-intrinsic mechanisms include overall tumor mutational burden and other tumor genomic factors. High tumor mutational burden increases neoantigen expression and surface antigen presentation for recognition by activated cytotoxic T-lymphocytes, augmented with the use of immune checkpoint inhibition. Tumor cell mutation of specific oncogenic drivers or signaling pathways results in altered responses to interferon gamma and reduced presentation of neoantigens on major histocompatibility complex (MHC) I, ultimately altering immune effector recruitment and activation. TCR (T-cell receptor); PD-1 (programmed cell death protein-1); PD-L1 (programmed death-ligand 1); CTLA-4 (cytotoxic T-lymphocyte antigen 4); MAPK (mitogen activated protein kinase); STAT (signal transducer and activator of transcription); JAK (Janus kinase); TGFβR (transforming growth factor beta receptor); INFγR (interferon gamma receptor); P (phosphorylated).