Fig. 4: Identification of ERFEVPFQ peptide by mass spectrometry and hepcidin repression by recombinant ERFEVPFQ protein.
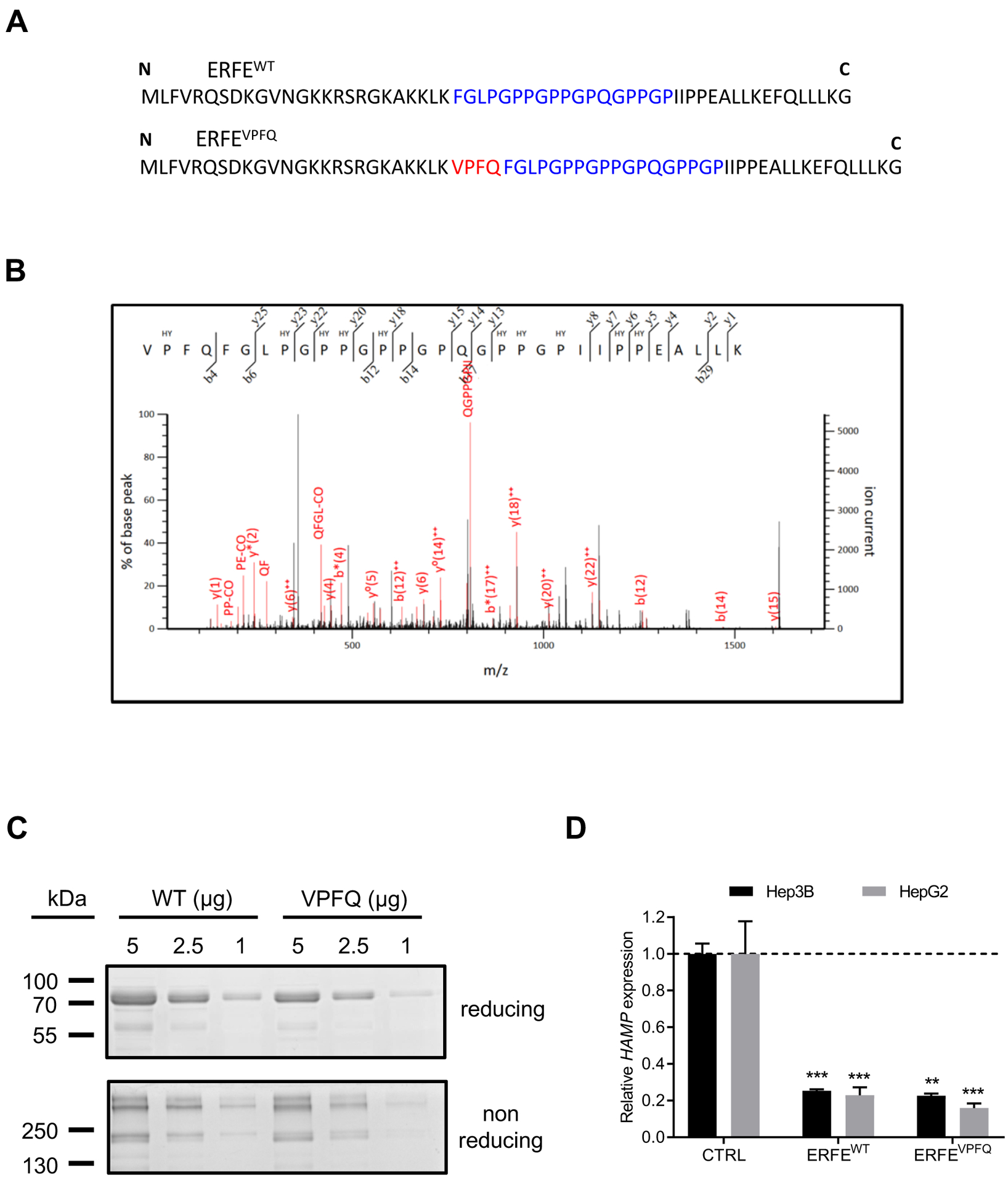
(A) Amino-acid sequence of ERFEWT and ERFEVPFQ peptides. VPFQ (red), collagen domain (blue). (B) Identification of a specific ERFEVPFQ peptide in erythroblast cell lysates by mass spectrometry using nano liquid chromatography coupled with a Q-Exactive Plus mass spectrometer. The calculated peptide mass was 3227.650782 from 3+ ion observed at m/z 1076.890870 with measured Δ = 3.6 ppm, Mascot Score = 17, expectation value = 0.069. HY: hydroxylated proline residues. (C) SDS-PAGE and Coomassie Blue staining of purified ERFEWT and ERFEVPFQ in reducing and non-reducing conditions. (D) Hep3B and HepG2 hepatocellular carcinoma cells were treated with 2 μg/ml of purified recombinant human ERFEWT or ERFEVPFQ for 16 hours. HAMP was quantified by RT-qPCR, and normalized to HPRT. Data shown are means ± SEM of three independent experiments and represent a fold change of hepcidin mRNA expression in ERFE-treated compared to untreated (CTRL) cells. Two-tailed Student t-test for P-values; *** P <0.0001; ** P <0.001.
