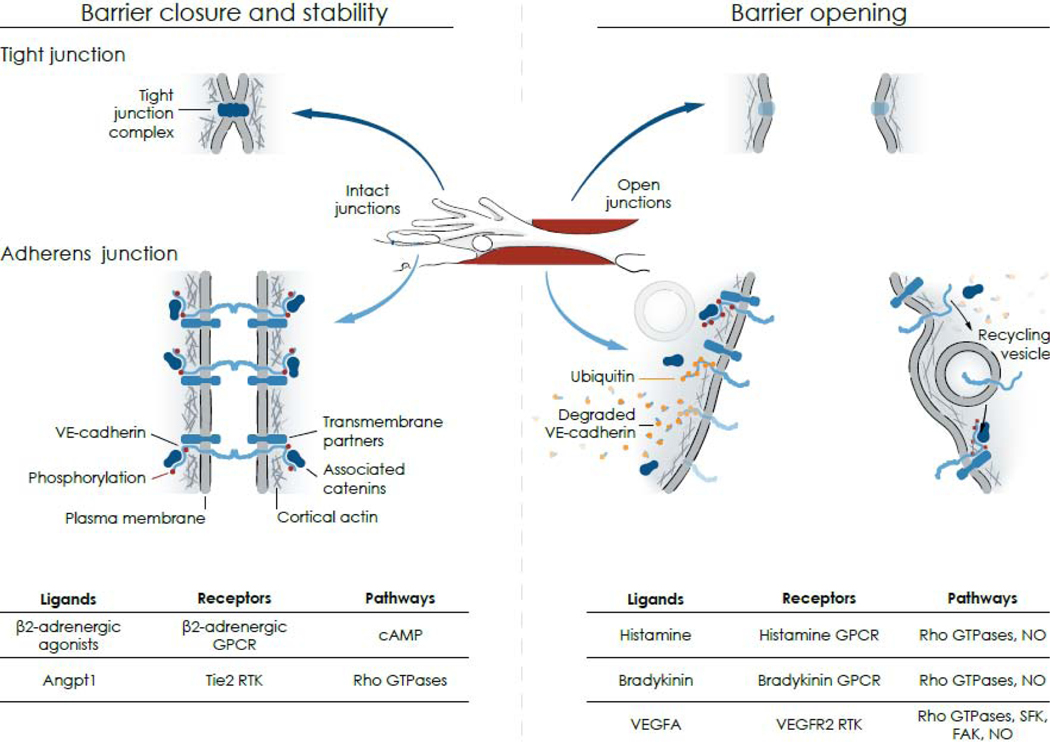
Figure 3. Mechanisms of barrier opening and closure.
Center: Drawing based on Figure 2 showing intact junctions (blue) between finger-like endothelial filopodia and an adjacent endothelial cell (left, Intact junctions) and a gap (red) between the same endothelial cells (right, Open junctions). Left arrows point to corresponding regions of intact tight junctions and adherens junctions; right arrows point to open junctions. Left: Intact endothelial cell barrier formed by homophilic interactions of tight junction complexes (upper) and adherens junction proteins (lower) at endothelial cell borders. At tight junctions, the fused outer leaflets of plasma membranes of adjacent cells create a diffusion barrier. At adherens junctions, VE-cadherin is in complex with catenins and other transmembrane proteins that provide structural attachments. Tyrosine residues of the intracellular domain of VE-cadherin have a low level of flow-dependent phosphorylation (few red dots) in endothelial cells of postcapillary venules at baseline. Right: Open barrier at endothelial gap resulting from changes in tight junctions (upper), which must still be defined, and adherens junctions (lower), where tyrosine phosphorylation of VE-cadherin increases (many red dots) followed by ubiquitination (orange dots), dismantling of complexes and degradation or internalization into vesicles and recycling back to the plasma membrane where complexes and tyrosine phosphorylation are restored. Bottom table: Ligands, receptors, and pathways implicated in barrier closure and stability (left) and barrier opening (right): Angpt1, angiopoietin 1; cAMP, cyclic adenosine monophosphate; FAK, focal adhesion kinase; GPCR, GTPase protein coupled receptor; NO, nitric oxide; RTK, receptor tyrosine kinase; SFK, Src family kinase; VEGFA, vascular endothelial growth factor.