Figure 2. Activation of oncogenes in GBM cells that affect GAM biology.
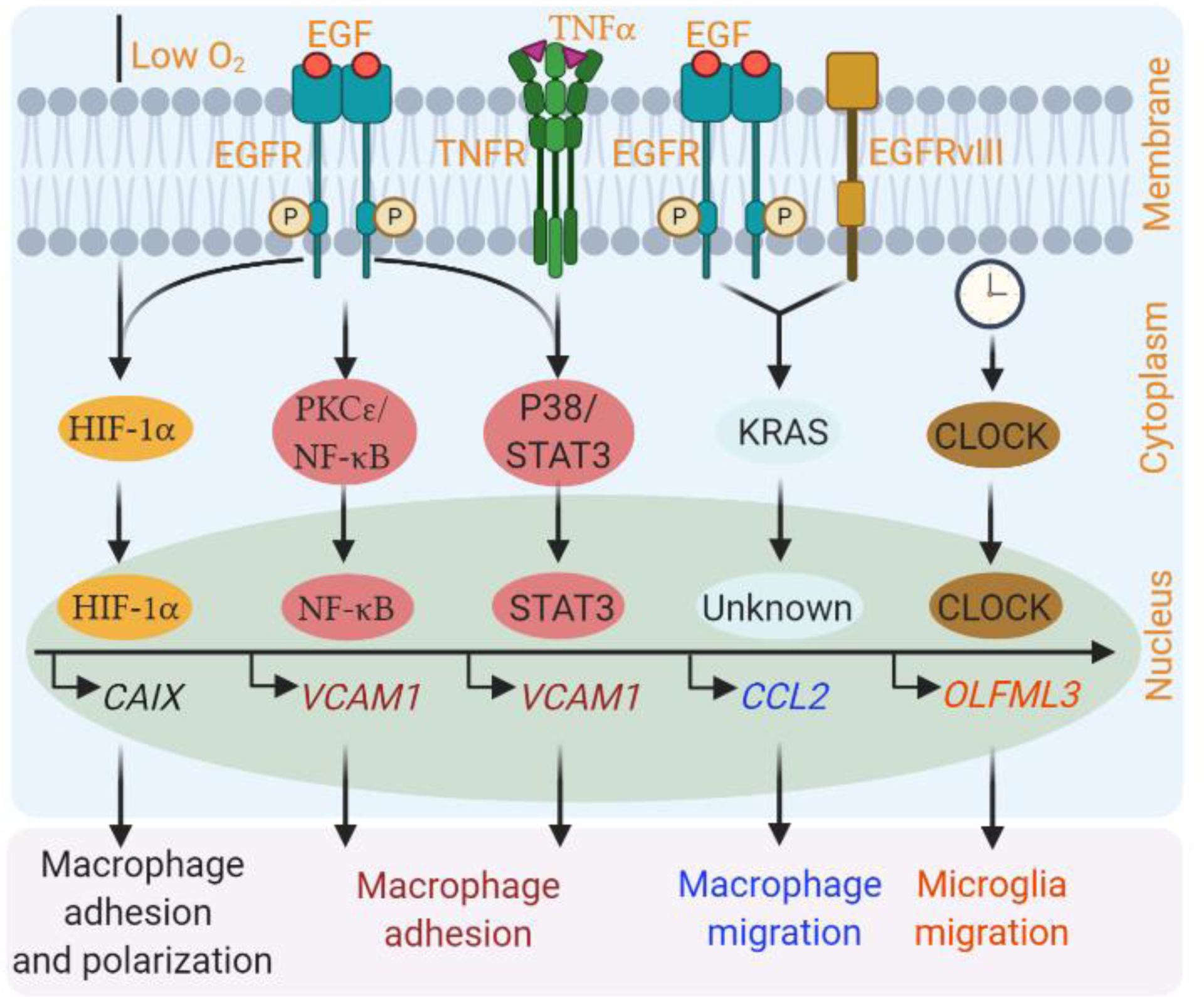
Activation of oncogenes (e.g., EGFR and CLOCK) in GBM cells can increase macrophage adhesion, migration and alternative polarization, as well as microglia migration through distinct mechanisms. For example, EGFR is required for hypoxia-induced activation of HIF-1α/CAIX axis in human GBM cells (e.g., U87 and U251), which can promote macrophage adhesion and alternative polarization [42]. In addition, EGFR is essential for EGF-induced activation of the PKCε/NF-κB pathway, and TNFα-induced activation of the P38/STAT3 axis in human and mouse glioma cells, which in turn, upregulate VCAM1 to increase macrophage adhesion [43]. EGFR can cooperate with EGFRvIII to upregulate KRAS, which in turn upregulates CCL2 to recruit macrophages. How CCL2 is regulated by KRAS in human GBM cells is still unknown [44]. CLOCK can transcriptionally upregulate chemokine OLFML3 in GSCs, which in turn recruits microglia into the GBM TME in mouse and human models [48]. This figure was created using BioRender (https://biorender.com/).
CAIX, carbonic anhydrase IX; CCL2, CC chemokine ligand 2; HIF-1α, EGF, Epidermal growth factor; EGFR, EGF receptor; EGFRvIII, EGFR variant III; GAM, glioblastoma-associated macrophage/microglia; GBM, glioblastoma; hypoxia-inducible factor 1-alpha; NF-κB, Nuclear factor kappa B; OLFML3, olfactomedin-like 3; PDX, patient-derived xenograft; PKCε, protein kinase C epsilon type, STAT3, signal transducer and activator of transcription 3; TNFα, tumor necrosis factor alpha; VCAM1, vascular cell adhesion molecule 1.
