Abstract
Despite advances in treatment, finding a cure for HIV remains a top priority. Chronic HIV infection is associated with increased risk of comorbidities such as diabetes and cardiovascular disease. Additionally, people living with HIV must remain adherent to daily antiretroviral therapy (ART), since lapses in medication adherence can lead to viral rebound and disease progression. Viral recrudescence occurs from cellular reservoirs in lymphoid tissues. In particular, lymph nodes are central to the pathology of HIV due to their unique architecture and compartmentalization of immune cells. Understanding how antiretrovirals (ARVs) penetrate lymph nodes may explain why these tissues are maintained as HIV reservoirs, and how they contribute to viral rebound upon treatment interruption. In this report, we review i) the physiology of the lymph nodes and their function as part of the immune and lymphatic systems, ii) the pathogenesis and outcomes of HIV infection in lymph nodes, and iii) ARV concentrations and distribution in lymph nodes, and the relationship between ARVs and HIV in this important reservoir.
Keywords: AIDS, antiretroviral, distribution, HIV, inflammation, physiology
Introduction
In the three decades since the first antiretroviral (ARV) was approved, antiretroviral therapy (ART) has transformed HIV infection from inevitable immune collapse and death into a chronic disease that can now be treated—and prevented—with one pill taken once per day.1 Despite these advances, finding a cure is paramount; long-term HIV infection is associated with increased risk for non-AIDS related cancers, cardiovascular diseases, diabetes mellitus, and other noninfectious comorbidities.1–4 Furthermore, although ART suppresses viral replication below detectable concentrations in blood plasma, treatment interruption can lead to viral rebound and disease progression.5–9
Rebound viremia originates from cellular reservoirs such as latently infected CD4+ T cells and macrophages,8,10 which can be found in anatomical sites like the central nervous system, gut-associated lymphoid tissue (GALT), or lymph nodes.7,9,11–14 ARV uptake and penetration into lymph nodes has not been well characterized, despite the fact that these tissues are repeatedly found to contain viral RNA and DNA in both nonhuman primates, humanized mice, and humans on ART.14–21 Lymph nodes are central to the pathology of HIV; they contain large populations of HIV-targeted CD4+ T cells and other immune cells organized in specific regions of the tissue to facilitate immune response.22–24 This unique internal structure also affects fluid flow and cellular transport, and may therefore influence the distribution of ARVs throughout the lymph node and their spatial relationship with HIV-infected cells.24–26
Here, we review the role of the lymph nodes in the lymphatic and immune systems, the pathogenesis of HIV in the lymph nodes, and summarize reports of ARV penetration into this important tissue reservoir. We also discuss research on the distribution of ARVs and HIV within the lymph node, and the implications of this important spatial relationship.
Overview of the Lymphatics
Physiology and functions of the lymphatic system
The primary function of the lymphatic system is to maintain fluid balance in the body by returning excess fluid to the bloodstream; in doing so, the lymphatics are a convenient highway for immune cells and thus a central component of the immune system.24,27 The lymphatic system consists of capillaries, vessels, lymph nodes, trunks, and ducts, and the fluid contained within the system is referred to as lymph. Unlike the closed-loop circulatory system, the lymphatic system is unidirectional, and begins with lymphatic capillaries that are interwoven within the circulatory capillary beds in the body’s tissues. Lymphatic capillaries can be 10–60 μm in diameter, and are comprised of a single, overlapping layer of endothelial cells, which are tethered to the surrounding extracellular matrix via anchoring filaments.24,28 The overlapping “flaps” of endothelial cells open with increases in interstitial fluid pressure and close again after fluid has been taken up into the lymphatic capillary and pressure has equalized.24
Following initial uptake, lymph flows from capillaries into collecting vessels. These vessels are comprised of a single layer of endothelial cells wrapped in smooth muscle and contain one-way bicuspid valves that regulate lymph movement and prevent back flow. All lymphatic vessels pass through at least one lymph node (covered in the next section) and converge to form lymphatic trunks. Lymphatic trunks drain into lymphatic ducts, which return lymph into the circulation. Six trunks empty into the thoracic duct at various points along its length, while three trunks empty into the right lymphatic duct. The thoracic duct drains trunks from the left and lower halves of the body, and returns lymph into the left subclavian vein, while the right lymphatic duct drains only the upper right side of the body into the right subclavian vein.27
Physiology and functions of the lymph node
While the cervical lymph nodes are most commonly known—especially during cold and flu season—there are 500–600 of these small (1–10 mm), bean-shaped sacs throughout the body.27 In the simplest sense, a lymph node is a region of fibrous tissue that has been encased by the stretched walls of a lymphatic vessel (Figure 1A). The endothelial cells that make up the vessel wall become the lymph node capsule, and the entering and exiting vessel segments become the afferent and efferent lymphatics, respectively. The point where the efferent lymphatics leave the lymph node is called the hilus; this is also where arterioles and venules enter and leave the lymph node. The lymph node tissue is divided into several lobules, with transverse sinuses running between each. As lymph moves from the afferent to the efferent vessels, it first flows into the outermost subcapsular sinus, continues between the lobules through the transverse sinuses, and finally flows into the lobules themselves through paracortical and medullary sinuses.29
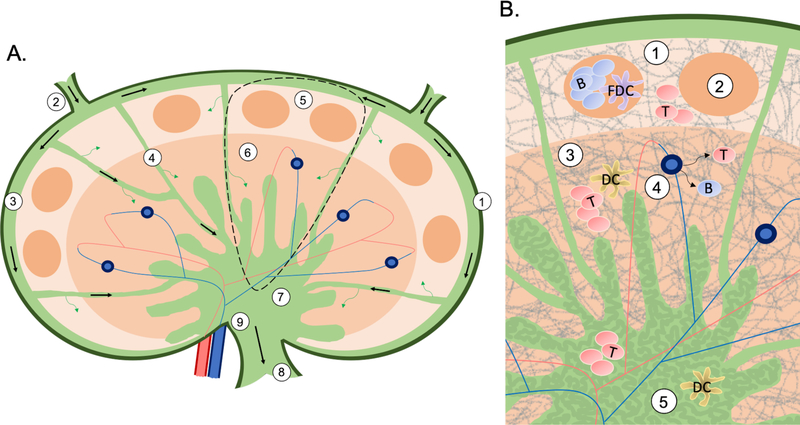
Figure 1:
A) An overview of the structure and flow (arrows) in a lymph node. The capsule (1) is the outmost later of endothelial cells. Lymphatic fluid flows into the node through the afferent (2) lymphatics, around the node through the subcapsular sinus (3), then between each lymph node lobule (dashed region, expanded in B) through the transverse sinuses (4). From the subcapsular sinus fluid can diffuse into the cortex (5), and from the transverse sinuses into the paracortex (6). Finally, lymphatic fluid collects in the medulla (7), and makes its way out of the node through the efferent lymphatics (8) in the hilus (9). B) An overview of the structure and cellular organization of a lymph node lobule. The cortex (1) is the outermost region containing follicles (2), which are made up primarily of B cells and FDCs. The next region is the paracortex (3), containing primarily T cells and DCs, as well as the HEVs (4). Lastly, the medulla (5) is an area of lymph flow and cell trafficking, and its appearance is characterized by a maze-like appearance of medullary cords (dark green) sinuses (light green). Throughout the lymph node the FRC network (gray lines) gives the tissue shape and structure.
Within each lobule, the tissue is organized into three regions that differ in their architecture and cell populations (Figure 1B). The outermost region closest to the afferent lymphatics is the cortex, which contains structures called follicles. Follicles are almost exclusively made up of B cells that interact with their antigen presenting cells (APCs), the follicular dendritic cells (FDCs).29–31 Surrounding the follicles is the interfollicular cortex containing primarily T cells, and specialized blood vessels known as high endothelial venules (HEVs).29,32 HEVs are also found in the middle region of the lobule, called the paracortex. The paracortex contains the paracortical sinuses, and a dense population of T cells and their APCs, the dendritic cells (DCs). Finally, the region closest to the hilus is the medulla. The medulla is an area of lymphocyte, fluid, and blood transit, with lymphatic fluid flowing out of the node through the medullary sinuses, and blood vessels entering the node within the medullary cords. The contrast of cell-dense cords and cell-sparse sinuses gives the medulla a maze-like appearance. Giving structural support to the entire lymphoid lobule is the reticular network created by fibroblastic reticular cells (FRCs).29 This network provides a dense “interstate” system for lymphocytes to crawl along as they search for their APCs, or as they migrate to follicles. In fact, the follicles and central paracortex are the only regions where this reticular network is scarce.
The distinct structure and cellular organization of the lymph node is key to effective immune response. The first step in this cascade is antigen presentation within the lymph node, which can occur via small (<70 kDa) antigens like soluble viral proteins, large (>70 kDa) antigens like bacteria or viral particles, or antigen-activated DCs.33 These antigens and APCs are taken up into lymphatic vessels within the body’s tissues, and are quickly carried to the nearest lymph node. Once DCs flow into the subcapsular sinus from the afferent vessel, they migrate across the sinus along chemokine gradients (CCL21, CCL19), which eventually lead the DCs to the paracortex. Meanwhile, naïve B and T cells migrate into the lymph node through HEVs.29,32 These cells also follow chemokine gradients which allow them to cross the walls of the HEVs, and crawl along the FRC network to the paracortex (T cells: CCL21, CCL19) and follicles (B cells: CXCL12, CXCL13). In the paracortex, the DCs present their antigen to naïve T cells; when a T cell recognizes its specific antigen, cell proliferation is triggered, causing production of millions of daughter cells and enlargement of the paracortex.29 Some of these cells, now called T-helper (Th) cells, migrate once again along the FRC network to the follicles. Within the follicles, naïve B cells have also been activated via antigen presentation by FDCs. At the perimeter of the follicle, the antigen-specific Th cells and antigen-activated B cells come into contact for the first time.29–32 Here, the Th cell signals the B cell to move to the center of the follicle forming a germinal center. In the germinal center, B cells mutate, proliferate, and undergo affinity selection in the “dark zone,” and receive differentiation signals from FDCs and T-follicular helper (Tfh) cells in the “light zone.”30,31,34 B cells will eventually undergo apoptosis, differentiate into memory B cells, or become plasmablasts that produce antibodies.31
As we have reviewed so far, the location of lymph nodes along lymph vessels and their specialized internal physiology make these tissues critical components of both the lymphatic and immune systems. Unfortunately, these distinct features also place the lymph node at the epicenter of HIV infection.
Pathogenesis of HIV within the Lymph Node
Effects of HIV infection on lymph nodes
At the start of the AIDS epidemic, lymphadenopathy was one of the first identified symptoms of HIV infection.35,36 Follicular structural changes caused by lymphadenopathy were later used to classify HIV progression into four stages: 1) follicular hyperplasia, 2) follicular lysis, 3) follicular atrophy, 4) follicular/lymphocytic depletion.37,38 In this final stage, CD4+ T cells are progressively depleted, leading to AIDS.
Progression across these stages results in CD4+ T cell depletion can occur though several mechanisms, including direct cell killing, indirect chronic immune activation, and inflammation-induced tissue fibrosis.39–44 Fibrosis is caused by collagen deposition that replaces normal tissue, and disrupts the careful organization of the lymph node. One of the most affected structures is the FRC network. FRCs and naïve CD4+ T cells are codependent, each producing chemokines that the other needs to survive; FRCs are the main source of IL-7 needed by naïve CD4+ T cells, while T cells produce lymphotoxin-β required by FRCs.39,45,46 Fibrosis restricts their ability to interact, leads to depletion of both cell types, and further limits T cell trafficking on a now diminished FRC super-highway. Taken together, these individual outcomes result in an overall suppression of immune response and disease progression within the lymph node.39–44 This fibrotic damage prevents adequate repopulation of CD4+ T cells and causes long-lasting structural changes in lymph nodes, even after ART is initiated.42,47
HIV infection and reservoir establishment
Following initial infection, HIV spreads to regional lymph nodes within 3–6 days due to their large population of HIV-targeted CD4+ T cells and constant cell trafficking by HEVs and lymphatic vessels; systemic dissemination occurs within 6–25 days.11,48 Previous studies suggest that the lymph node reservoir may be established within the first 2 weeks of infection: Kline et al. found strong correlation between concentrations of viral DNA in monkey lymphoid tissue and plasma viral load one week post-infection, while Bourry et al. found similar levels of viral DNA in lymphoid tissues from untreated monkeys and those on ART.49–51 During acute infection, large numbers of virions are attached to FDCs, and productively infected cells can be found throughout the lymph node (Figure 2A);14,18 imaging analyses of viral burden in various nonhuman primate organs prior to ART initiation show that the lymph nodes contain 36% of HIV RNA+ cells.18 After ART is initiated, concentrations of HIV RNA in plasma and lymph nodes (on FDCs and in productively infected mononuclear cells) decrease dramatically within 2–22 days,52 but the virus is not completely eradicated.11–18,49,53 HIV RNA has been extracted and quantified from lymph node tissue—and imaged in mononuclear cells—after 2 years of suppressive ART,15,54 and HIV DNA is frequently found in the lymph nodes of individuals with HIV suppressed in plasma.15,18,54 Upon treatment interruption, reactivation of latently infected cells, or new infections caused by FDC-trapped virions, can lead to rebound viremia throughout the body.5–7,12
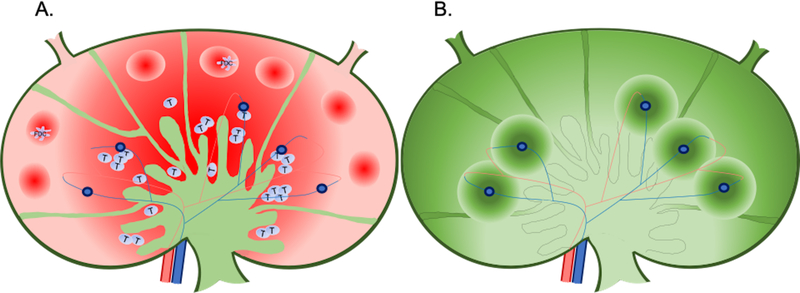
Figure 2:
An overview of expected A) HIV and B) ARV distribution in the lymph node. A) The red color gradient represents variability in HIV distribution throughout the lymph node, with more virus located in dark regions and less virus in lighter regions. Higher HIV concentrations are expected the the B cells follicles, where virions are trapped on FDCs, and in the T cell zone in infected CD4+ T cells. B) The green color gradient represents changes in drug distribution throughout the lymph node, with higher drug concentrations in dark regions and lower drug concentrations in lighter regions. Increased ARV penetration is expected around the subcapsular and transverse sinuses, and surrounding HEVs.
There are two main theories of how the lymph node reservoir is maintained during ART: low-level viral replication,17,53,55–57 or clonal expansion of latently infected CD4+ T cells.7,49,58–61 Ongoing viral production and infection in tissue is thought to be caused by low ARV concentrations compared to those in plasma, resulting in incomplete inhibition of viral replication in the lymph node.18,19,55 Newly infected cells may stay in the lymph node or be trafficked into circulation, but in either case replication remains far below the limits of detection of current assays, leading to the belief that complete suppression has been achieved.55,56 The theory of ongoing replication is supported by phylogenetic analyses of evolving viral sequences first isolated from lymph nodes and later found in PBMCs,55 and by imaging analyses of virus-producing cells within lymph nodes and other tissues during ART.18,19
In the case of clonal expansion, lymph node CD4+ T cells are infected prior to ART, and then become quiescent.62 Once treatment is initiated, these latently infected cells can undergo proliferation, clonally expanding into cell populations with identical viral sequences, which can be trafficked from lymph nodes to blood and other tissues.7,58,60,61,63,64 These latent clones can become stochastically reactivated,65 and if treatment is interrupted or drug concentrations are too low to prevent new infections, rebound of viruses with identical sequences can occur from multiple anatomical compartments.7,61,63 Evidence supporting the theory of clonal expansion is based on phylogenetic analyses showing that identical viral sequences in lymph nodes and blood are from proliferating clones rather than multiple-cell infection with one viral species,63 and that viral evolution during ART is not significantly different from sequences obtained prior to treatment.61
Lymph Nodes and ART
Overview of lymphatic penetration of ARVs and other drugs
Orally administered drugs are absorbed in the enterocyte, and enter systemic circulation through transport into portal blood or the intestinal lymphatics; this process is governed by several factors. First, although small-molecule drugs can readily diffuse into both blood and lymph capillaries, the intestinal flow rate of blood is about 500-times higher than that of lymph, so the majority of small-molecule drugs are preferentially taken up into the bloodstream from the enterocyte.22,66 However, favorable physicochemical properties (higher molecular weights, logP > 5, large particle size, >50 mg/mL solubility in long-chain triglycerides) can increase orally administered drug transport via the lymph.
Increased lymph targeting is also achieved through advanced lipid-based formulations, including emulsions, liposomes, and nanoparticles. The majority of nanoparticle or liposomal formulations are delivered subcutaneously to take advantage of direct lymphatic uptake66; well-known examples of subcutaneous liposomal drugs include the antineoplastic agents doxorubicin and paclitaxel.67,68 Subcutaneous administration of particles 10–100 nm in diameter results in preferential uptake into the leakier lymphatic capillary, and exclusion from the tight endothelial junctions of the blood capillary.66 These strategies are currently being employed with ARVs, which traditionally do not have favorable physicochemical properties for intestinal lymphatic transport after oral administration. Table 1 compares in vivo data from several studies investigating lymph node penetration after subcutaneous or topical delivery of liposomal and nanoparticle ARV formulations, along with the molecular weights and log P values of the unmodified free drugs (further discussion of in vitro studies of investigational ARV formulations, ARV-targeting of other reservoir tissues, and long-acting formulations in plasma can be found in Shao et al.68, Gao et al.69, and Edagwa et al.70). The studies listed in Table 1 demonstrate that subcutaneous nanoparticle and liposomal ARVs can result in increased drug concentrations in lymph nodes compared to the original small-molecule formulations.71–75
Table 1:
Lymph node targeting of ARV liposomal/nanoparticle formulations
| ARV | Drug class | Free drug Log P | Free drug molecular weight (g/mol) | Free drug route of administration | Mean free drug concentration in lymph nodes (ng/g) or LNMCs (ng/mL) | Nanoparticle/ liposomal route of administration | Mean liposomal/ nanoparticle drug concentration in lymph nodes (ng/g) or LNMCs (ng/mL) | % increase | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AZT | NRTI | 0.05 | 267.24 | Topical | 50 | Topical | 1500 | 2,900 | 75 |
| IDV | PI | 3.3 | 613.8 | PO | 0.2 | SubQ | 320 | 160,000 | 71 |
| LPV | PI | 5.9 | 628.8 | SubQ | 0 | SubQ | 1212 | - | 74 |
| RTV | PI | 6 | 720.9 | SubQ | 33 | SubQ | 1642 | 4876 | |
| TFV | NRTI | −1.6 | 287.2 | SubQ | 256 | SubQ | 190 | −26 | |
| ATV | PI | 4.5 | 704.9 | SubQ | 21 | SubQ | 63 | 200 | 72 |
| RTV | PI | 6 | 720.9 | SubQ | ND | SubQ | 252 | - | |
| TFV | NRTI | −1.6 | 287.2 | SubQ | 32 | SubQ | 37 | 16 | |
| TFV | NRTI | −1.6 | 287.2 | SubQ | - | SubQ | 4217 | - | 73 |
| 3TC | NRTI | −1.4 | 229.3 | SubQ | - | SubQ | 2144 | - | |
| LPV | PI | 5.9 | 628.8 | SubQ | - | SubQ | 1597 | - | |
| RTV | PI | 6 | 720.9 | SubQ | - | SubQ | 1064 | - | |
PO: oral; SubQ: subcutaneous; LNMC: lymph node mononuclear cell; PBMC: peripheral blood mononuclear cell; AZT: zidovudine; IDV: indinavir; LPV: lopinavir; RTV: ritonavir; TFV: tenofovir; ATV: atazanavir; 3TC: lamivudine; PI: protease inhibitor; NRTI: nucleoside/nucleotide reverse transcriptase inhibitor; ND: not detected; -: not assessed
ARV distribution throughout the lymph node
Once in the bloodstream or lymph, ARVs are delivered to the lymph node (Figure 2B). Table 2 describes possible regions of drug localization in the lymph node based on the physicochemical properties of common ARVs.76 Protein-unbound drug in the plasma may diffuse across HEV walls into the lymph node parenchyma, or be taken up by naïve T cells in blood and trafficked into the lymph node via cell migration through HEVs. After drug enters the lymph node by diffusion and/or intracellular transport across HEV walls, it can perfuse throughout the T cell zone or be further trafficked (via Th cells) to B cell follicles.66 We would expect that ARVs arriving at the lymph node via the plasma would have transient residence in the T cell zone, and may diffuse quickly back into blood. Nanoparticle and liposomal ARV formulations aim to improve drug retention in the lymph node and limit drug diffusion back into the blood through increased particle size, similar to nanochemotherapeutics designed to exploit the enhanced permeability and retention (EPR) effect when targeting tumors.77
Table 2:
Likely region of common antiretroviral distribution within lymph node, based on physicochemical properties
| Drug class | ARV | Log P | Molecular weight (g/mol) | Water solubility (mg/mL) | Lymph node region |
|---|---|---|---|---|---|
| NRTI | Abacavir | 1.2 | 286.33 | 1.21 | Subcapsular sinuses, possibly cortex, area immediately surrounding HEVs |
| Emtricitabine | −0.43 | 247.25 | 112 | ||
| Lamivudine | −1.4 | 229.26 | 70 | ||
| Tenofovir | −1.6 | 287.21 | 13.4 | ||
| NNRTI | Efavirenz | 4.6 | 315.68 | 0.00855 | Deeper central tissue: paracortex, medulla |
| Rilpivirine | 4.86 | 366.42 | <0.1 | ||
| Doravirine | 3.51 | 425.75 | 0.00321 | ||
| PI | Atazanavir | 4.5 | 704.86 | 0.00327 | Paracortex, medulla |
| Darunavir | 1.89 | 547.66 | 0.15 | Periphery, cortex, area around HEVs | |
| INSTI | Bictegravir | 1.28 | 449.39 | 0.0537 | Periphery, cortex, area around HEVs |
| Dolutegravir | 2.2 | 419.38 | 0.0922 | ||
| Raltegravir | 0.4 | 444.42 | 5.4e-7 | ||
| Elvitegravir | 3.66 | 447.88 | 0.00652 |
Cortex, paracortex |
|
NRTI: nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; INSTI: integrase inhibitor; all data from DrugBank
ARVs taken up into the intestinal lymphatics (those with logP > 5, increased lipid solubility, etc.) are packaged into lipid vesicles in the enterocyte and carried by lymph fluid flow to the node. Protein-unbound ARVs may also be taken up by memory T cells in lymph and carried into the lymph node. ARVs entering the node within lipid particles, or intracellularly, likely distribute based on lymph flow patterns; 90% of afferent lymph quickly flows through the subcapsular and medullary sinuses, while 10% slowly perfuses into the central parenchyma.26 Lipophilic ARVs will be more likely to perfuse into the middle of the lymph node, where there is high cellular density and low fluid flow rates. Cells containing ARVs may be carried along the periphery of the node via the path of fluid flow, or utilizing the FRC network to migrate to the T cell zone.
ARV distribution throughout the lymph node may also be affected by the tissue fibrosis discussed in the previous section. Collagen fibrosis reduces T cell populations and disrupts the FRC network, therefore limiting the quantity and motility of cells available for intracellular drug trafficking. Cell depletion would likely have the greatest impact on nucleoside/nucleotide reverse transcriptase inhibitor (tenofovir, emtricitabine, lamivudine, etc.) distribution in the lymph node, since these drugs have the least favorable physicochemical properties for free drug perfusion (low molecular weight, low lipophilicity, fast diffusion back into blood) and likely distribute most effectively through cell trafficking. Furthermore, inflammation and fibrosis can alter the structure and size of the lymph node, disrupting normal pressure gradients and drug perfusion patterns throughout the tissue.26,78,79 Jagarapu et al. found an association between increased lymphoid lobule size and drug exclusion from the center of the lobule.79 This is consistent with Jafarnejad et al.’s findings that lymphadenopathy during inflammation and/or viral infection can cause higher pressure in the lymph node and result in increased fluid flow back into blood vessels.26,80
Quantifying ARV concentrations and distribution in the lymph node
Table 3 provides several examples of ARV concentrations quantified in lymph node tissue or isolated lymph node mononuclear cells (LNMCs).19,50,71,81,82 Bourry et al. examined ARV concentrations in cells isolated from lymph nodes of 5 macaques treated with zidovudine, lamivudine, and indinavir 14–28 days post infection with simian immunodeficiency virus (SIV). Lamivudine concentrations were 25 fold lower in lymph node cells than in peripheral blood mononuclear cells (PBMCs), and zidovudine concentrations were below limits of quantitation in the lymph node.50 Fletcher et al. assessed intracellular ARV concentrations in lymph node mononuclear cells (LNMCs) from 12 HIV+ human donors on regimens of tenofovir/emtricitabine plus either efavirenz, atazanavir/ritonavir, or darunavir/ritonavir. Similar to Bourry et al., Fletcher found that ARV concentrations were 66–100% lower in LNMCs than PBMCs.19 A study by Solas et al. showed conflicting results in lymph node homogenate obtained from 41 HIV+ donors on suppressive ART regimens: nelfinavir, lopinavir, and ritonavir penetration into lymph nodes was 21–64% of what it was in plasma, while indinavir penetration was 207% greater in lymph nodes.81 Our laboratory has examined lymph node homogenate from 13 HIV+ donors on emtricitabine, tenofovir, efavirenz, raltegravir, and atazanavir, and found that lymph node penetration was similar to or greater than plasma, with tissue:blood plasma penetration ratios ranging from 0.8 to 2.3.82
Table 3:
Reported antiretroviral concentrations in lymph nodes and plasma
| Tissue processing method | ARV | LN concentration | Plasma concentration | Reported difference | Reference |
|---|---|---|---|---|---|
| Homogenized LN biopsy | IDV | 1,025 ng/g tissue | 321 ng/mL | LN:plasma ratio: 2.07 | 81 |
| NFV | 740 ng/g tissue | 1,328 ng/mL | LN:plasma ratio: 0.58 | ||
| LPV | 1,260 ng/g tissue | 7,333 ng/mL | LN:plasma ratio: 0.21 | ||
| RTV | 410 ng/g tissue | 641 ng/mL | LN:plasma ratio: 0.64 | ||
| LNMCs isolated from LN biopsy | IDV | 0.12–0.6 ng/5×105 LNMCs | 0.53–1.72 ng/5×105 PBMCs 616 ng/mL (plasma) |
LNMC:PBMC ratio: 0.23–0.35 | 71 |
| Cells isolated from LN tissue | 3TC | 0.006 pmol/106 cell equivalents | 0.15 pmol/106 cell equivalents | Not reported | 54 |
| ZDV | BLQ | Not reported | |||
| IDV | Not reported | Not reported | |||
| LNMCs isolated from minced, extruded LN biopsy | TFVdp | 10–30* fmol/106 LNMCs | 70–100* fmol/106 PBMCs 47.4 ng/mL (plasma, parent drug) |
LNMC:PBMC ratio: 0.1–0.43 | 19 |
| FTCtp | 1,000–3,000* fmol/106 LNMCs | 5,000–9,000* fmol/106 PBMCs 63.1 ng/mL (plasma, parent drug) |
LNMC:PBMC ratio: 0.11–0.6 | ||
| ATV | BLQ | 1,000–3,000* fmol/106 PBMCs 377 ng/mL (plasma) |
LNMC:PBMC ratio: BLQ | ||
| DRV | BLQ-100* fmol/106 LNMCs | 1,000–8,000* fmol/106 PBMCs 1,310 ng/mL (plasma) |
LNMC:PBMC ratio: BLQ-0.1 | ||
| EFV | 20–1,000* fmol/106 LNMCs | 1,000–2,000* fmol/106 PBMCs 1,750 ng/mL (plasma) |
LNMC:PBMC ratio: 0.01–1 | ||
| Homogenized LN section | FTC | 12.9 ng/g tissue | 17.5 ng/mL | LN:plasma ratio: 1.2 | 82 |
| TFV | 282 ng/g tissue | 74.9 ng/mL | LN:plasma ratio: 1.0 | ||
| EFV | 5,041 ng/g tissue | 445 ng/mL | LN:plasma ratio: 2.3 | ||
| RAL | 263 ng/g tissue | 21.3 ng/mL | LN:plasma ratio: 1.5 | ||
| ATV | 1,679 ng/g tissue | 1,450 ng/mL | LN:plasma ratio: 0.8 | ||
LN: lymph node; BLQ: below limit of quantitation; LNMC: lymph node mononuclear cell; PBMC: peripheral blood mononuclear cell; IDV: indinavir; NFV: nelfinavir; LPV: lopinavir; RTV: ritonavir; 3TC: lamivudine; ZDV: zidovudine; TFV(dp): tenofovir (diphosphate); FTC(tp): emtricitabine (triphosphate); ATV: atazanavir; DRV: darunavir; EFV: efavirenz; RAL: raltegravir
data estimated from figure in reference
The variability in these results likely reflects differences in sampling methods between research groups (shown in Table 3). There are two main methods of determining dug exposure in tissue: measuring drug concentrations in cells isolated from tissue, or directly in tissue homogenates; Table 4 summarizes these processing methods and the pros and cons of each. While both methods have been used reliably, results can vary due to differences in tissue processing,83 and neither method accounts for nonuniform drug distribution in tissue.84,85 In lymph nodes, LNMCs isolated from a fine-needle biopsy may be sampled from a region of the lymph node where drug is not distributed, thus underestimating true drug exposure. Conversely, if concentrations are measured in lymph node homogenate, they represent the average drug exposure over that entire piece of lymph node tissue, and may overestimate drug exposure in unexposed tissue regions. The variability of drug exposure in tissue highlights the importance of investigating not only ARV concentration within the lymph node and other HIV reservoirs, but the distribution of these drugs as well.
Table 4:
Advantages and disadvantages of various methods of analyzing antiretrovirals in tissue
| Tissue processing method | Pros | Cons |
|---|---|---|
| LC-MS/MS and Tissue Homogenization | • Minimize drug lost during processing • Homogenate is sampled and analyzed directly • Enough homogenate for one sample to be used for several analyses (LC-MS/MS, protein binding, RNA/protein expression, etc.) |
• Loss of regional drug exposure information (concentration averaged) • Mixture of intracellular and extracellular components • If not controlled for, homogenization may introduce heat, affecting drug stability |
| Enzymatic Digestion and Cell Isolation | • Quantifies cell-associated concentrations where drug is active • Removes any extracellular particles • Minimal blood contamination |
• Less sample material • Drug may be lost during isolation • Loss of regional drug exposure information (cells/biopsy taken from one location, do not represent the whole tissue) • If not controlled for, serial washes, centrifugations, buffers can degrade sample and result in lower concentrations |
| Traditional Drug Imaging (Quantitative Whole-body Autoradiography, Positron Emission Tomography) | • In vivo drug imaging • Provides spatial distribution • Images the entire 3-D volume of distribution |
• Relies on radiolabeling, which is not compatible with all drugs/compounds • May not distinguish between parent drug and metabolite(s) • Resolution is low • Simultaneous analysis of multiple drugs is challenging • Does not provide an absolute quantitation of drug concentration |
| Mass Spectrometry Imaging | • Provides spatial distribution • Can distinguish between parent drug and metabolites • Able to evaluate multiple drugs in one analysis, and correct for background |
• Ex vivo drug measurements • Only images drug in one 2-D cross-section at a time • Resolution is low • Unless a calibration curve is used, quantitation is not absolute |
Mass spectrometry imaging (MSI) has been used to investigate the within-tissue distribution of many analytes including proteins and endogenous biomarkers.86,87 To overcome the limitations of the sampling modalities mentioned above, MSI methods have been developed to quantify ARV concentrations while simultaneously preserving spatial data.88–93 Thompson et al. was the first to investigate ARV distribution within several reservoir tissues from simian/human immunodeficiency virus (SHIV) infected macaques, and found heterogeneous efavirenz distribution throughout cross-sections of lymph nodes; the maximum ARV signal intensity detected in a lymph node section was up to 15-fold higher than the minimum intensity.94 Similar results were found in other reservoir tissue including the colon (max/min intensity fold difference: 37.6), ileum (7.5), spleen (14.6), and brain (5.8 to 14.5).94,95 Ntshangase et al. performed qualitative MSI of tenofovir and elvitegravir in the brains of rats, and found preferential distribution of elvitegravir into the thalamus, hypothalamus, and corpus callosum; subsequent investigations by the same group also showed heterogeneity in emtricitabine and efavirenz distribution in rat brains, though neither of these studies quantified ARV concentrations in brain regions to determine the true extent of heterogeneity.90,96
To fully understand how this ARV variability affects HIV persistence, it is crucial to investigate the overlap between ARVs and HIV expression in these sites. Furthermore, the location and concentration of drug proximate to virus must be determined to understand the potential true efficacy of ARVs within tissues. To this end, the combination of quantitative MSI with microscopy provides some insight into the overlap of effective ARV concentrations with various targets of interest., including viral RNA or DNA (in situ hybridization), immune cells or collagen deposition (immunohistochemistry), or lymph node morphology (H&E staining).97 Thompson et al. colocalized MSI distribution of 6 ARVs with viral RNA and CD3+ T cell images in tissue sections taken from the gut of SHIV+ macaques, HIV+ humanized mice, and HIV+ humans.98 Across all species, ARV distribution was heterogeneous and drugs did not colocalize consistently to target cells or viral RNA expression: 50–60% of CD3+ T cells and 90% of viral RNA was not exposed to inhibitory drug concentrations.98 Taken together, these data illustrate the importance of ARV concentration and distribution to viral persistence in the lymph node and other putative HIV reservoirs.
Conclusion
Lymph nodes are one of the most important tissue reservoirs of the virus. Their distinct cellular compartmentalization creates an environment well-suited to HIV infection, pathogenesis, dissemination, and latency. HIV infection and immune activation in lymph nodes leads to fibrotic tissue damage that exacerbates immunosuppressive consequences such as CD4+ T cell depletion and inflammation. Initiation of ART reduces viral burden in plasma and lymph nodes, but HIV is not fully cleared from lymph nodes, which may be caused by inadequate ARV penetration throughout the tissue. Indeed, heterogeneous drug distribution in lymph nodes and other implicated tissue reservoirs has been demonstrated, and nonuniform drug coverage of virus—or ARV coverage at subtherapeutic concentrations—provides support for a pharmacologic contribution to viral reservoir persistence. It is necessary to continue researching ARV pharmacology in the lymph node reservoir to improve the efficacy of current treatments and develop future therapies to eradicate HIV.
Acknowledgments
Funding: National Institutes of Health Grant R01 AI111891
Footnotes
Conflict of Interest statement: All authors declared no competing interests for this work.
References
- 1.Cihlar T & Fordyce M Current status and prospects of HIV treatment. Curr Opin Virol 18, 50–56 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Guaraldi G et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 53, 1120–1126 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Guaraldi G et al. Cost of noninfectious comorbidities in patients with HIV. Clinicoecon. Outcomes Res. 5, 481–488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffau P et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS 32, 1651–1660 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Li JZ et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 30, 343–353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin R et al. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 30, 761–769 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Vibholm LK et al. Characterization of intact proviruses in blood and lymph node from HIV-infected individuals undergoing analytical treatment interruption. J. Virol. (2019).doi: 10.1128/JVI.01920-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abreu CM et al. Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann N et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat. Commun. 10, 3193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrager LK & D’Souza MP Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 280, 67–71 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Wong JK & Yukl SA Tissue reservoirs of HIV. Curr Opin HIV AIDS 11, 362–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiike M et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 423, 107–118 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88, 9838–9842 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deleage C et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog. Immun. 1, 68–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamers SL et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J. Virol. 90, 8968–8983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury G et al. Human Immunodeficiency Virus Persistence and T-Cell Activation in Blood, Rectal, and Lymph Node Tissue in Human Immunodeficiency Virus-Infected Individuals Receiving Suppressive Antiretroviral Therapy. J. Infect. Dis. 215, 911–919 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North TW et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84, 2913–2922 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes JD et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 23, 1271–1276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher CV et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 111, 2307–2312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton PW & García JV Humanized mouse models of HIV infection. AIDS Rev 13, 135–148 (2011). [PMC free article] [PubMed] [Google Scholar]
- 21.Marsden MD et al. HIV latency in the humanized BLT mouse. J. Virol. 86, 339–347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher CV & Podany AT Antiretroviral Drug Penetration into Lymphoid Tissue. In Encyclopedia of AIDS (Hope TJ, Stevenson M & Richman D) 1–9 (Springer New York, New York, NY, 2014).doi: 10.1007/978-1-4614-9610-6_436-1 [DOI] [Google Scholar]
- 23.Dimopoulos Y, Moysi E & Petrovas C The lymph node in HIV pathogenesis. Curr HIV/AIDS Rep 14, 133–140 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Swartz MA The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 50, 3–20 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Hay JB & Hobbs BB The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J. Exp. Med. 145, 31–44 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafarnejad M, Woodruff MC, Zawieja DC, Carroll MC & Moore JE Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes. Lymphat Res Biol 13, 234–247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.OpenStax Chapter 21. The Lymphatic and Immune System. In Anatomy and Physiology 137–144 (OpenStax CNX, 2013).at <https://opentextbc.ca/anatomyandphysiology/chapter/21-1-anatomy-of-the-lymphatic-and-immune-systems/> [Google Scholar]
- 28.Schmid-Schönbein GW Microlymphatics and lymph flow. Physiol. Rev. 70, 987–1028 (1990). [DOI] [PubMed] [Google Scholar]
- 29.Willard-Mack CL Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 34, 409–424 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Mesin L, Ersching J & Victora GD Germinal center B cell dynamics. Immunity 45, 471–482 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Silva NS & Klein U Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girard J-P, Moussion C & Förster R HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Liao S & Weid PY von der Lymphatic system: an active pathway for immune protection. Semin. Cell Dev. Biol. 38, 83–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannard O et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity 39, 912–924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez R, Mouradian J, Metroka C & Davis J The prognostic value of histopathology in persistent generalized lymphadenopathy in homosexual men. N. Engl. J. Med. 309, 185–186 (1983). [DOI] [PubMed] [Google Scholar]
- 36.Metroka CE et al. Generalized lymphadenopathy in homosexual men. Ann. Intern. Med. 99, 585–591 (1983). [DOI] [PubMed] [Google Scholar]
- 37.Vago L et al. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl. Pathol. 7, 298–309 (1989). [PubMed] [Google Scholar]
- 38.Baroni CD & Uccini S Lymph nodes in HIV-positive drug abusers with persistent generalized lymphadenopathy: histology, immunohistochemistry, and pathogenetic correlations. Prog AIDS Pathol 2, 33–50 (1990). [PubMed] [Google Scholar]
- 39.Estes JD Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol. Rev. 254, 65–77 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng M et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 121, 998–1008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schacker TW et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 13, 556–560 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estes JD, Haase AT & Schacker TW The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin. Immunol. 20, 181–186 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacker TW et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110, 1133–1139 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estes JD et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195, 551–561 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Katakai T, Hara T, Sugai M, Gonda H & Shimizu A Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng M, Haase AT & Schacker TW Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 33, 306–314 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Schacker TW et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J. Infect. Dis. 186, 1092–1097 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Cohen MS, Shaw GM, McMichael AJ & Haynes BF Acute HIV-1 Infection. N. Engl. J. Med. 364, 1943–1954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kline C et al. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One 8, e84275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourry O et al. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology 7, 78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leyre L et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci. Transl. Med. 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavert W et al. Kinetics of Response in Lymphoid Tissues to Antiretroviral Therapy of HIV-1 Infection. Science 276, (1997). [DOI] [PubMed] [Google Scholar]
- 53.Rose R et al. HIV Maintains an Evolving and Dispersed Population in Multiple Tissues during Suppressive Combined Antiretroviral Therapy in Individuals with Cancer. J. Virol. 90, 8984–8993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Günthard HF et al. Residual human immunodeficiency virus (HIV) Type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J. Infect. Dis. 183, 1318–1327 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Lorenzo-Redondo R et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530, 51–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Natarajan V et al. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Highly active antiretroviral therapy. Lancet 353, 119–120 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Buzón MJ et al. Deep molecular characterization of HIV-1 dynamics under suppressive HAART. PLoS Pathog. 7, e1002314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simonetti FR et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl. Acad. Sci. USA 113, 1883–1888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kearney MF et al. Ongoing HIV replication during ART reconsidered. Open Forum Infect. Dis. 4, ofx173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coffin JM et al. Clones of infected cells arise early in HIV-infected individuals. JCI Insight (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McManus WR et al. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J. Clin. Invest. 130, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohn LB et al. HIV-1 integration landscape during latent and active infection. Cell 160, 420–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hosmane NN et al. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 214, 959–972 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller RL et al. HIV Diversity and Genetic Compartmentalization in Blood and Testes during Suppressive Antiretroviral Therapy. J. Virol. 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahabieh MS, Battivelli E & Verdin E Understanding HIV latency: the road to an HIV cure. Annu. Rev. Med. 66, 407–421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trevaskis NL, Kaminskas LM & Porter CJH From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 14, 781–803 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Ali Khan A, Mudassir J, Mohtar N & Darwis Y Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int. J. Nanomedicine 8, 2733–2744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao J et al. Nanodrug formulations to enhance HIV drug exposure in lymphoid tissues and cells: clinical significance and potential impact on treatment and eradication of HIV/AIDS. Nanomedicine (Lond.) 11, 545–564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Y, Kraft JC, Yu D & Ho RJY Recent developments of nanotherapeutics for targeted and long-acting, combination HIV chemotherapy. Eur J Pharm Biopharm 138, 75–91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edagwa BJ, Zhou T, McMillan JM, Liu X-M & Gendelman HE Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr. Med. Chem. 21, 4186–4198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinman L et al. Lipid-drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J. Acquir. Immune Defic. Syndr. 34, 387–397 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Perazzolo S et al. Three HIV Drugs, Atazanavir, Ritonavir, and Tenofovir, Coformulated in Drug-Combination Nanoparticles Exhibit Long-Acting and Lymphocyte-Targeting Properties in Nonhuman Primates. J. Pharm. Sci. 107, 3153–3162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McConnachie LA et al. Long-Acting Profile of 4 Drugs in 1 Anti-HIV Nanosuspension in Nonhuman Primates for 5 Weeks After a Single Subcutaneous Injection. J. Pharm. Sci. 107, 1787–1790 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freeling JP, Koehn J, Shu C, Sun J & Ho RJY Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res. Hum. Retroviruses 31, 107–114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jain S, Tiwary AK & Jain NK PEGylated elastic liposomal formulation for lymphatic targeting of zidovudine. Curr. Drug Deliv. 5, 275–281 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Wishart DS et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 36, D901–6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golombek SK et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 130, 17–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagai T, Ikomi F, Suzuki S & Ohhashi T In situ lymph dynamic characterization through lymph nodes in rabbit hind leg: special reference to nodal inflammation. J Physiol Sci 58, 123–132 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Jagarapu A, Piovoso MJ & Zurakowski R An Integrated Spatial Dynamics-Pharmacokinetic Model Explaining Poor Penetration of Anti-retroviral Drugs in Lymph Nodes. Front. Bioeng. Biotechnol. 8, 667 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jafarnejad M et al. Quantification of the whole lymph node vasculature based on tomography of the vessel corrosion casts. Sci. Rep. 9, 13380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solas C et al. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47, 238–243 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgunder E et al. Antiretroviral Drug Concentrations in Lymph Nodes: A Cross-Species Comparison of the Effect of Drug Transporter Expression, Viral Infection, and Sex in Humanized Mice, Nonhuman Primates, and Humans. J. Pharmacol. Exp. Ther. 370, 360–368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cory TJ, Winchester LC, Robbins BL & Fletcher CV A rapid spin through oil results in higher cell-associated concentrations of antiretrovirals compared with conventional cell washing. Bioanalysis 7, 1447–1455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischman AJ et al. Pharmacokinetics of [18F]trovafloxacin in healthy human subjects studied with positron emission tomography. Antimicrob. Agents Chemother. 42, 2048–2054 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Müller M, Peña A. dela & Derendorf H Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48, 1441–1453 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miura D, Fujimura Y & Wariishi H In situ metabolomic mass spectrometry imaging: recent advances and difficulties. J. Proteomics 75, 5052–5060 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Groseclose MR, Andersson M, Hardesty WM & Caprioli RM Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J Mass Spectrom 42, 254–262 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Prideaux B & Stoeckli M Mass spectrometry imaging for drug distribution studies. J. Proteomics 75, 4999–5013 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Bokhart MT et al. Quantitative mass spectrometry imaging of emtricitabine in cervical tissue model using infrared matrix-assisted laser desorption electrospray ionization. Anal. Bioanal. Chem. 407, 2073–2084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ntshangase S et al. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: Application of MALDI-MSI and LC-MS/MS. Rapid Commun. Mass Spectrom. (2019).doi: 10.1002/rcm.8510 [DOI] [PubMed] [Google Scholar]
- 91.Bokhart MT & Muddiman DC Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging analysis of biospecimens. Analyst 141, 5236–5245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barry JA et al. Mapping antiretroviral drugs in tissue by IR-MALDESI MSI coupled to the Q Exactive and comparison with LC-MS/MS SRM assay. J Am Soc Mass Spectrom 25, 2038–2047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robichaud G, Barry JA & Muddiman DC IR-MALDESI mass spectrometry imaging of biological tissue sections using ice as a matrix. J Am Soc Mass Spectrom 25, 319–328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson CG et al. Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob. Agents Chemother. 59, 2944–2948 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Srinivas N et al. Antiretroviral concentrations and surrogate measures of efficacy in the brain tissue and CSF of preclinical species. Xenobiotica 49, 1192–1201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ntshangase S et al. Mass Spectrometry Imaging Demonstrates the Regional Brain Distribution Patterns of Three First-Line Antiretroviral Drugs. ACS Omega 4, 21169–21177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van de Plas R, Yang J, Spraggins J & Caprioli RM Image fusion of mass spectrometry and microscopy: a multimodality paradigm for molecular tissue mapping. Nat. Methods 12, 366–372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson CG et al. Heterogeneous antiretroviral drug distribution and HIV/SHIV detection in the gut of three species. Sci. Transl. Med. 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]