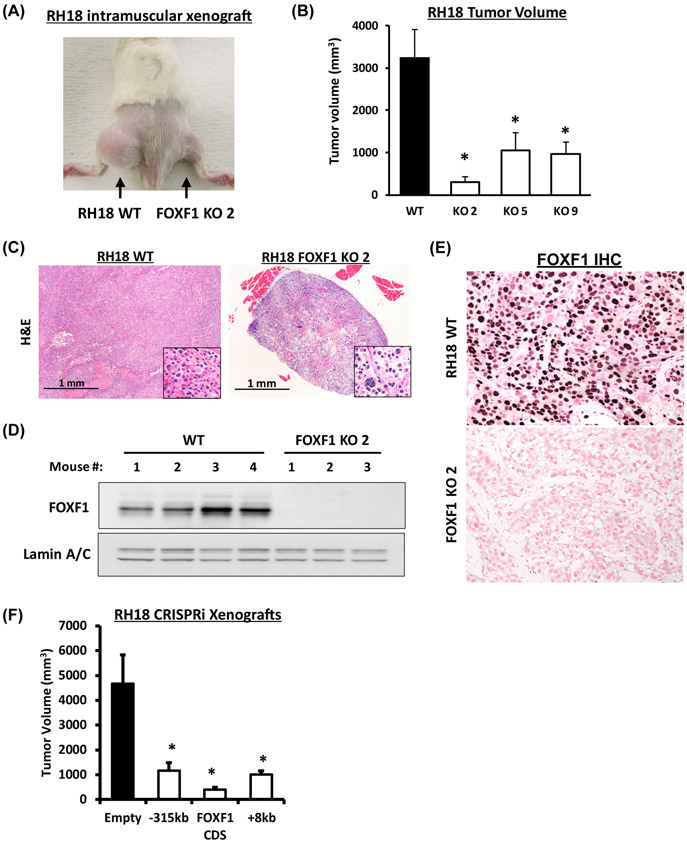
Figure 3. FOXF1 is required for PAX3-FOXO1 to drive FP-RMS tumor growth.
(A) Xenograft tumors eight weeks after intramuscular inoculation of parental (RH18 WT) tumor cells into left flank or FOXF1-depleted RH18 (FOXF1 KO) tumor cells into right flank. (B) Tumor volumes were measured eight weeks after tumor cells inoculation. RH18 WT xenografts n=8; FOXF1KO xenografts using clones 2, 5, 9, n=4 per each cell line. Data reported as mean ± SEM. (C) H&E staining of FP-RMS tumors developed from parental RH18 WT and from FOXF1 KO tumor cells. (D) Western blot analysis of tumor lysates shows high levels of FOXF1 protein in WT RH18 tumors and complete loss of FOXF1 protein in FOXF1 KO tumors. Lamin A/C was used as a loading control. (E) Immunohistochemistry shows the expression of FOXF1 in parental RH18 WT tumors, but not in the FOXF1 KO tumors. (F) Repression of either −315kb upstream or +8kb downstream enhancers using CRISPRi inhibited FP-RMS tumor growth in orthotopic mouse model. RH18 cells were transduced with a lentivirus containing a dCas9:KRAB and a gRNA targeting either −315kb upstream or +8kb downstream enhancers. Repression of FOXF1 coding sequence (CDS) was used as a positive control. Generated stable cell lines were injected into the quadriceps of NSG mice and sacrificed after eight weeks (n=6 per group).