Abstract
Comorbidity of tuberculosis (TB) and depression may lead to delayed TB treatment initiation. A cross sectional study was conducted between January and December 2019 to examine the association between depression and delayed TB treatment initiation among newly diagnosed TB patients in Botswana. We used the Patient Health Questionnaire-9 and the ZUNG self-rating anxiety scale to assess depressive and anxiety symptoms, respectively. Delayed TB treatment was defined as experiencing common TB symptoms for more than 2 months before treatment initiation. We used Poisson regression models with robust variance to assess the association between covariates and delayed treatment initiation. Majority of the enrolled 180 study participants were males (n =116, 64.4%). Overall, 99 (55%) were co-infected with HIV; depression and anxiety symptoms were reported by 47.2% and 38.5% of the participants respectively. The prevalence of delayed TB treatment was 42.6% and 18.8% among participants who indicated symptoms of depression and among participants without depression respectively. After adjusting for age, HIV status, gender and anxiety symptoms, depression was still associated with delayed TB treatment (adjusted prevalence ratio [aPR] = 2.09; 95% CI = 1.23–3.57). Integrating management of depressive symptoms during TB treatment may help in improving overall TB treatment outcomes.
Keywords: Anxiety, Mental illness, Common mental disorders, HIV, TB/HIV comorbidity
Introduction
Tuberculosis is the leading cause of morbidity and mortality due to an infectious disease worldwide accounting for 2.0% of the global disease burden (Murray et al., 2012; World Health Organisation, 2019b). Botswana has one of the highest burdens of TB per capita in the world, the notification rate being 275 per 100,000 population (World Health Organisation, 2019a).
Depression is the most common comorbid psychiatric disorder among patients with TB (Ambaw et al., 2017; Doherty et al., 2013). A World Health Survey conducted in 48 LMICs reported that the prevalence of depressive episodes among those with and without TB was 23.7% and 6.8%, respectively (Koyanagi et al., 2017). However, depression is often under recognised because of the overlap of the core symptoms of TB and depression. Moreover, clinicians may attribute depressive symptoms to normal stress reactions to being diagnosed with TB (Sweetland et al., 2017). Previous research has reported depression rates ranging from 40% to 60% among African populations of patients with TB (Ambaw et al., 2017; Dasa et al., 2019; Ige & Lasebikan, 2011; Kehbila et al., 2016). A recently published scoping review on comorbidities between tuberculosis and common mental disorders reported only three studies conducted in Southern Africa on rates of depression among TB patients (Janse Van Rensburg et al., 2020). The reported prevalence rates were 9.3% in Zambia (van den Heuvel et al., 2013), 11.35% in South Africa (Tomita et al., 2019) and 49.4% in Angola (Xavier & Peixoto, 2015).
Early detection and treatment of TB patients is the cornerstone of the end TB strategy (Asres et al., 2018). Varied prevalence rates of delay in TB diagnosis and treatment have been reported in low- and middle-income countries. Rates ranging from 46% to 96% have been reported in Ethiopia (Wondawek & Ali, 2019), 48% in Zimbabwe (Takarinda et al., 2015), and 20.6% in India (Das et al., 2017). Delays in diagnosing and treating TB results in severe symptoms at treatment initiation which may lead to poor treatment outcomes including mortality and drug resistance (Asres et al., 2018). Furthermore, patients whose treatment is delayed remain infectious for long with consequent onward transmission and increase in the number of cases (Ambaw et al., 2017). Treatment delay has also been reported as a contributing factor to the high mortality rate among patients co-infected with HIV (Segagni Lusignani et al., 2013).
Depression is a significant predictor of delayed TB treatment; symptoms of depression such as avolition, social isolation and indecisiveness may negatively affect health seeking behaviour of patients with TB and depression leading to delayed diagnosis and treatment initiation (Koyanagi et al., 2017; Trivedi & Greer, 2014).
The relationship between TB and depression may be bidirectional (Koyanagi et al., 2017). The production of pro inflammatory cytokines and weakened immunity which occurs in depression may increase vulnerability of patients to developing TB (Kiecolt-Glaser & Glaser, 2002; Oh et al., 2017). Depression may also present with neglected self-care which poses a risk factor for acquiring TB (Koyanagi et al., 2017). On the other hand, depression may develop as a psychological response to the diagnosis of TB and the stigma it carries; the inflammatory response to Mycobacterium tuberculosis increases the risk of developing depression (Koyanagi et al., 2017).
Although several studies have been conducted on TB treatment or diagnostic delay (Awoke et al., 2019; Bojovic et al., 2018; Li et al., 2013; Storla et al., 2008; WHO, 2006) including in Botswana (Steen & Mazonde, 1998), the role of depression hasn’t been adequately explored. Most research conducted on the comorbidity of TB and depression has been on risk factors and treatment outcomes such as adherence and loss to follow up. There is paucity of data on depression and its association with delayed TB treatment initiation which may be a major contributing factor to prognosis of treatment. The objective of our study was to examine the association between depression and treatment delay among newly diagnosed TB patients.
Materials and Methods
Study design and site description
This was an institutional-based cross-sectional study, conducted from January to December 2019 at 12 primary health care facilities in Gaborone, Botswana. Botswana is an upper middle-income country in Southern Africa with an active TB incidence of 275/100 000 (World Health Organisation, 2019a). Gaborone, the capital and largest city in Botswana has a population of approximately 280,000 (Statistics Botswana, 2015). The facilities included in the study were purposively selected based on high TB incidence (Ministry of Health and Wellness, 2018).
Study population
The target population was patients attending the selected facilities, aged 18 years or above and newly diagnosed with TB. Patients who had just been diagnosed with TB were identified in the lab and TB clinic register.
Patients who required urgent medical care, or had difficulty with hearing, speaking, or understanding both English and Setswana, were excluded from the study.
Measures
Researcher designed socio-economic and demographic questionnaire
The instrument was used to capture data such as gender, income, employment status, history of alcohol use and smoking.
Patient Health Questionnaire (PHQ-9)
The PHQ-9 was used to screen for depressive symptoms based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association (APA), 2000). It is a nine-item instruments where respondents endorse frequency of depression symptoms in the preceding two weeks on a 4-point likert scale. Kroenke et al. reported that total scores of 10 or more is indicative of depression (Kroenke et al., 2001). The PHQ-9 has demonstrated excellent sensitivity (88%), specificity (88%) and internal reliability (Cronbach’s α of 0.89) when used in a primary care population (Kroenke et al., 2001). The internal reliability of the PHQ-9 in the current study was acceptable with a Cronbach’s alpha of 0.74.
Zung self-rating anxiety scale (ZUNG)
The ZUNG is a 20-item self-rating instrument which was used to assess for anxiety symptoms. The instrument assesses for cognitive, autonomic, motor and central nervous system symptoms of anxiety. Respondents indicate how much each statement applies to them within a period of one or two weeks prior to taking the test on a Likert-type scale ranging from “a little of the time,” “some of the time,” “good part of the time,” “most of the time” (1–4) (Zung, 1971). The ZUNG demonstrated good internal reliability with a Cronbach’s alpha of 0.80 in our study.
Data Collection Procedures
Data were collected in one-time interviews of TB patients using standardized questionnaires. Research assistants were trained by MD and AHF to administer the English and Setswana versions of the questionnaires used in this study.
Potential participants identified daily from the lab and clinic register were approached by Research Assistants (RA) for recruitment. RA met the patients in a private room and screened them for the eligibility criteria before conducting the consent process. A written informed consent was obtained before commencing with data collection. RAs read consent materials in English and/or Setswana to patients who were illiterate; all participants verified their consent to participate by signature.
Data on socioeconomic, demographic characteristics and clinical information was collected using indirect administration of questionnaires whereby the research interviewer asked questions and recorded response given by the participants. To improve accuracy of responses in mental health related questions, assisted direct administration of questionnaires was used, in which the questions were read by the interviewer and participants recorded their response on the tablet directly. This has been reported to reduce social desirability bias in mental health related interviews (Bowling, 2005).
Participants whose total score corresponded with mild depression (score 5–9) were given a contact sheet for psychiatric services so that they could seek help if symptoms worsened or persisted. Research assistants facilitated referrals by making appointments with mental health professionals for participants who scored 10 or more (moderate to severe depression) or those who presented with suicidal ideation. Facilitation of referral was done with the participants’ consent.
Data Analysis
In this study, the outcome variable was delayed TB treatment initiation, which was defined as experiencing common TB symptoms, such as fever, night sweats, and weight loss, for more than 2 months. The prevalence of treatment delay in a subgroup was calculated by dividing the number of participants with delayed treatment by the total number of participants in the subgroup. Participants were specified as having depression if they scored equal to or more than 10 points on PHQ-9, and as having anxiety if they scored equal to or more than 36 points on ZUNG. We used Poisson regression model with robust variance to estimate the independent effect of depression on TB treatment delay. This method has been recommended to analyze prevalence ratios for binary outcomes in cross-sectional studies (Zou, 2004). The independent covariates were selected based on a priori knowledge, which consist of age, HIV co-infection, gender, anxiety, and depression. All covariates were included in the final multivariable Poisson model regardless of their bivariate association with treatment delay. All data cleaning and analysis were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina, U.S.). There was no alpha cutoff specified in statistical analyses following recent statistical guidelines (Greenland et al., 2016).
Ethics
The study was approved by the institutional review board (IRB) at University of California, Irvine and Botswana Ministry of Health and Wellness, Health Research and Development Division (HPDME 13/18/1). Permission to carry out the study was also obtained from the district health management team and senior management of the respective primary health care facilities.
Results
Sociodemographic and clinical characteristics
A majority of the 180 participants in our study were male (64.4%, n= 116) and the mean age was 37years (Table 1). More than half (55%, n= 99) of the newly diagnosed TB patients were co-infected with HIV. Patients were more likely to be diagnosed with pulmonary TB (76.1%, n=137). Treatment delay was reported by 45 (25%) participants, the majority of which were male (65.1%) and HIV-positive (62.8%).
Table 1.
Participant characteristics
| Characteristic | No depressive symptoms n (%) or median (IQR) | Depressive symptoms n (%) or median (IQR) | All participants n (%) or median (IQR) | |
|---|---|---|---|---|
| Gender | Male | 90 (67.7) | 26 (55.3) | 116 (64.4) |
| Female | 43 (32.3) | 21 (44.7) | 64 (35.6) | |
| Age | 37.0 (28.0–45.0) | 38.0 (27.0–48.0) | 37 (27.5–46.0) | |
| Marital status | Married | 15 (11.3) | 3 (6.4) | 18 (10.0) |
| Single | 114 (85.7) | 41 (87.2) | 155 (86.1) | |
| Divorced/separated/widow | 4 (3.0) | 3 (6.4) | 7 (3.9) | |
| Education | None | 12 (9.0) | 5 (10.6) | 17 (9.4) |
| Secondary or less | 89 (66.9) | 34 (72.3) | 123 (68.3) | |
| Tertiary or certificate | 16 (12.0) | 3 (6.4) | 19 (10.6) | |
| Diploma or degree | 15 (11.3) | 5 (10.6) | 20 (11.1) | |
| Masters or PHD | 1 (0.8) | 0 | 1 (0.6) | |
| Income (in Botswana pula1) | No income | 46 (34.6) | 19 (40.4) | 65 (36.1) |
| < 5000 | 74 (55.6) | 24 (51.1) | 98 (54.4) | |
| 5000–10000 | 12 (9.0) | 2 (4.3) | 14 (7.8) | |
| > 10000 | 1 (0.8) | 2 (4.3) | 3 (1.7) | |
| TB diagnosis methods | Culture | 1 (0.8) | 1 (2.1) | 2 (1.1) |
| XPERT | 52 (39.1) | 20 (42.6) | 72 (40.0) | |
| AFB | 19 (14.3) | 4 (8.5) | 23 (12.8) | |
| Chest X-ray | 31 (23.3) | 8 (17.0) | 39 (21.7) | |
| Clinical | 30 (22.6) | 14 (29.8) | 44 (24.4) | |
| TB symptoms length | None | 6 (4.5) | 0 | 6 (3.3) |
| < 1 month | 69 (51.9) | 19 (40.4) | 88 (48.9) | |
| 1–2 months | 33 (24.8) | 8 (17.0) | 41 (22.8) | |
| 2–3 months | 10 (7.5) | 10 (21.3) | 20 (11.1) | |
| > 3 months | 15 (11.3) | 10 (21.3) | 25 (13.9) | |
| Smoking | No | 106 (79.7) | 29 (61.7) | 135 (75.0) |
| Yes | 27 (20.3) | 18 (38.3) | 45 (25.0) | |
| Alcohol | No | 79 (59.4) | 22 (46.8) | 101 (56.1) |
| Yes | 54 (40.6) | 25 (53.2) | 79 (43.9) | |
| Disease classification3 | Pulmonary | 105 (79.5) | 32 (68.1) | 137 (76.5) |
| Extrapulmonary | 27 (20.5) | 15 (31.9) | 42 (23.5) | |
| HIV infection | No | 63 (47.4) | 18 (38.3) | 81 (45.0) |
| Yes | 70 (52.6) | 29 (61.7) | 99 (55.0) | |
| Time since HIV diagnosis in months2 | 37.5 (1.0–123.0) | 73.0 (1.0–90.0) | 43.0 (1.0–119.0) | |
| CD4 T-cell count at time closest to TB diagnosis2 | 318.0 (105.0–438.0) | 405.5 (184.0–471.0) | 335.0 (120.0–469.0) | |
| ART history2 | Never taken ART | 24 (34.3) | 7 (24.1) | 31 (31.3) |
| Taking ART | 40 (57.1) | 19 (65.5) | 59 (59.6) | |
| Took ART but stopped | 6 (8.6) | 3 (10.3) | 9 (9.1) | |
| Anxiety | Normal (20–35) | 85 (63.9) | 10 (21.3) | 95 (52.8) |
| Minimal to moderate anxiety (36–47) | 47 (35.3) | 24 (51.1) | 71 (39.4) | |
| Marked to severe anxiety (48–59) | 1 (0.8) | 10 (21.3) | 11 (6.1) | |
| Most extreme anxiety (>=60) | 0 | 3 (6.4) | 3 (1.7) |
Abbreviations: ART=Antiretroviral therapy; IQR=Interquartile range; TB=tuberculosis; XPERT=GeneXpert MTB/RIF; AFB=Acid-Fast Bacilli smear; ART=antiretroviral therapy
5000 Botswana pula is approximately 465 US dollars
Among HIV-infected participants only
Does not add to n=180, one participant data missing
Marriage was protective against depressive symptoms in our sample as single participants experienced depressive symptoms more than those who were married (Table 1). Participants who were co-infected with HIV, had secondary or less education and earned a monthly income of below 5000 Botswana Pula (BWP) (465USD) were more likely to experience depressive symptoms than those who weren’t infected with HIV, had more than secondary education and earned above 5000 BWP respectively.
Occurrence of Depression and Anxiety symptoms among newly diagnosed TB patients
Total scores from the PHQ-9 and ZUNG are shown in Figure 1 and 2, respectively. Overall, 172 (95.6%) participants reported some depressive symptoms, and 47 (26.1%) scored greater than 10, suggesting a severity of depressive symptoms consistent with those of major depression. Significant anxiety symptomatology was reported by 85 participants (those who scored 36 or above).
Figure 1. Depressive symptomatology1 among participants.
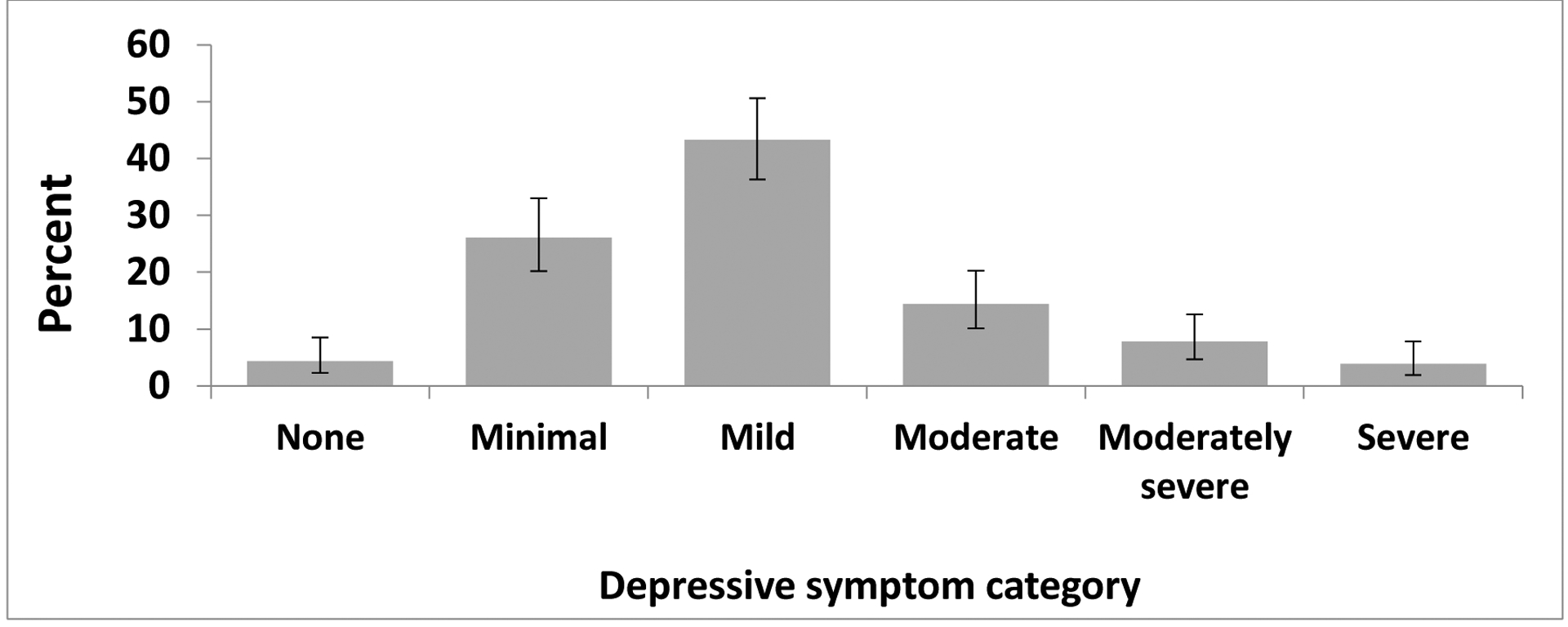
Note: Error bars are representative of 95% confidence limits calculated by the Wilson score method.
1 Depressive symptom categories are based on Patient Health Questionnaire (PHQ-9) scores: 0=None; 1–4=Minimal depression; 5–9=Mild depression; 10–14=Moderate depression; 15–19=Moderately severe depression; 20–27=Severe depression
Figure 2. Anxiety symptomatology1 among participants.
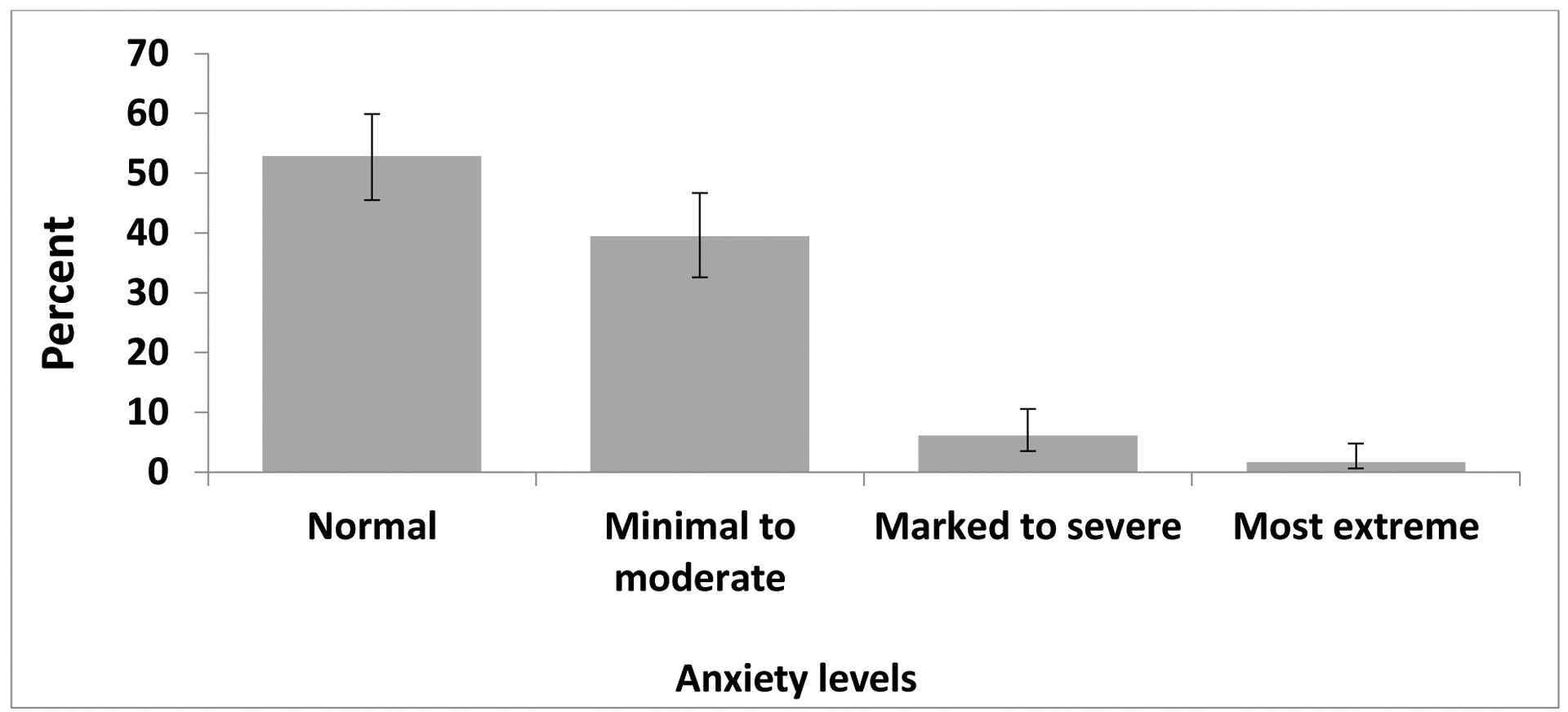
Note: Error bars are representative of 95% confidence limits calculated by the Wilson score method.
1 Anxiety symptom categories are based on Zung self-rating anxiety scale (ZUNG) raw scores: Below 36=Normal; 36–47=Minimal to moderate; 48–59=Marked to severe; 60 and over=Most extreme.
Delayed TB treatment
A total of 25% of the participants met the criteria of delayed TB treatment as defined in the current study (having TB symptom for more than 2 months before treatment initiation), with 13.9% having treatment delay longer than three months.
Depression and delayed TB treatment
The prevalence of TB treatment delay was 42.6% and 18.8% among participants who indicated symptoms of depression and among participants without depression, respectively (Table 2). In bivariate Poisson analysis, we found depression was associated with treatment delay (crude prevalence ratio [cPR] = 2.26; 95% confidence interval [CI] = 1.39, 3.68). After adjusting for age, HIV status, gender and anxiety, depression remained associated with higher prevalence of delayed TB treatment (adjusted prevalence ratio [aPR] = 2.09; 95% CI = 1.23–3.57).
Table 2.
Correlates of delayed TB treatment initiation1
| Characteristic | Prevalence n/N (%) | Crude PR (95% CI) | Adjusted PR (95% CI) | |
|---|---|---|---|---|
| Depression | No | 25/133 (18.8) | 1.00 | 1.00 |
| Yes (≥10 on PHQ9) | 20/47 (42.6) | 2.26 (1.39–3.68) | 2.09 (1.23–3.57) | |
| HIV status | Negative | 17/81 (21.0) | 1.00 | 1.00 |
| Positive | 28/99 (28.3) | 1.35 (0.80–2.28) | 1.17 (0.70–1.97) | |
| Age | N/A | 1.02 (0.99–1.03) | 1.01 (0.99–1.03) | |
| Gender | Male | 29/116 (25.0) | 1.00 | 1.00 |
| Female | 16/64 (25.0) | 1.00 (0.59–1.70) | 0.93 (0.55–1.58) | |
| Anxiety | No | 19/95 (20.0) | 1.00 | 1.00 |
| Yes (raw score ≥36 on ZUNG) | 26/85 (30.6) | 1.53 (0.91–2.56) | 1.13 (0.66–1.92) |
Delayed treatment initiation was defined as having TB symptom for more than 2 months
Discussion
Depression was found to be significantly associated with delayed treatment initiation among TB patients even after adjusting for potential confounders such as co-infection with HIV, anxiety symptoms and gender. Our findings contrast with findings from Ethiopia where they found that depression did not have a statistically significant association with diagnostic delay (Ambaw et al., 2019). The difference may be accounted for by differences in health services provided in the two countries. For example in Ethiopia, community health workers conduct home visits to encourage people with symptoms of TB to seek help, therefore the impact of depression in treatment delay is likely to be reduced (Ambaw et al., 2019); whereas in Botswana, patient diagnoses largely depend on them presenting to health care facilities. It may be worthwhile for the Botswana health sector to adapt the same model and utilise already existing lay health care workers such as health education officers to conduct home visits and screen for TB symptoms, this will hopefully reduce rates of delayed treatment among depressed patients.
Our findings complement evidence that medical conditions detection may be delayed in people with depression (Koyanagi et al., 2017). Patients with depression may experience hopelessness to a point of passively or actively wishing to die. The consequence of this hopelessness will be delays in help seeking when unwell with consequent delayed diagnosis and treatment. Moreover, delayed diagnosis and treatment may also result from other symptoms of depression such as avolition, chronic fatigue and difficulty making decisions. Indecisiveness is likely to result in patients postponing consultations for their TB symptoms.
The prevalence of depression in our study population is comparable to the average rate of depression found in other low and middle income countries (LMICs) (Koyanagi et al., 2017). The rates reported in TB patients are significantly higher than rates reported in the general population, further confirming reports of increased risk of depression in patients with chronic medical conditions (Wang et al., 2018).
Attention to depression as a factor in TB diagnosis and treatment has only recently begun. In contrast to past studies of the delay in diagnosis and treatment of TB that often failed to consider depression as a contributing factor for delayed TB diagnosis and treatment (Storla et al., 2008), more recent work has begun to recognise the impact of poor mental health on treatment outcomes for chronic conditions such as TB (Pachi et al., 2013; Patel et al., 2018).
Our findings highlight the need for ongoing depression screening and management during TB treatment. There is evidence from India and Peru that treating psychiatric manifestations of TB results in improved treatment adherence, completion and cure rates (Acha et al., 2007; Janmeja et al., 2005). Health care workers across all stages of health care services should be trained to recognize symptoms of depression among TB patients.
The strengths of our study include the inclusion of different health facilities and use of standardized questionnaires which have been shown to have good to excellent psychometric properties to assess for depression. To the best of our knowledge, this is one of the very few studies assessing the association between the comorbidity of TB and depression and association with treatment delay. We believe statistically adjusting for the association which may have been attributable to co-infection with HIV which has been reported as a significant predictor of treatment delay (Coimbra et al., 2012; Nogueira et al., 2018) strengthened our findings.
Our study is not without limitations. Our findings are not generalizable to severely sick and hospitalized TB patients because they were not included in our study. The cross-sectional design of the study limits our inference on the direction of causality. We did not collect data to discriminate between delays due to treatment seeking behavior by the patient and treatment delay secondary to health care facility factors. For example, some patients may have presented early to lower levels of care but took long to be diagnosed until referral to our study sites. Lastly, we cannot rule out the effect of recall bias on our findings because we relied on patients recall of onset of symptoms in order to determine treatment delay. Recollection of onset of TB symptoms might also be adversely affected by our exposure: symptoms consistent with major depression.
Conclusion and Recommendations
Depression predicts delayed TB treatment initiation. Delay in diagnosis negatively impacts the disease prognosis and increases risk of transmission within the community.
Our findings highlight the need for a multidisciplinary approach to the management of TB. Health care professionals who are diagnosing and treating TB patients should be aware of the co-existence of TB and depression and routinely screen for depression. Simultaneous treatment of TB and depression may be the answer to improve clinical outcomes of patients with TB and depression.
Previous research has demonstrated the clinical utility of treating the psychological aspects of TB (Acha et al., 2007; Janmeja et al., 2005), therefore, future research should focus on the development of effective and affordable interventions for treatment of depression among newly diagnosed TB patients in order to address the dual burden of TB and depression.
Acknowledgments
We are thankful to the study participants who made this study possible.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases under grant K01AI118559.
Footnotes
Declaration of interest statement
The authors have no potential conflict of interest to declare.
References
- Acha J, Sweetland A, Guerra D, Chalco K, Castillo H, & Palacios E (2007). Psychosocial support groups for patients with multidrug-resistant tuberculosis: Five years of experience. Global Public Health, 2(4), 404–417. 10.1080/17441690701191610 [DOI] [PubMed] [Google Scholar]
- Ambaw F, Mayston R, Hanlon C, & Alem A (2017). Burden and presentation of depression among newly diagnosed individuals with TB in primary care settings in Ethiopia. BMC Psychiatry, 17(1). 10.1186/s12888-017-1231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambaw F, Mayston R, Hanlon C, & Alem A (2019). Is depression associated with pathways to care and diagnosis delay in people with tuberculosis in Ethiopia? Global Mental Health, 6. 10.1017/gmh.2019.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA). (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. In American Psychiatric Association. 10.1176/appi.books.9780890423349 [DOI] [Google Scholar]
- Asres A, Jerene D, & Deressa W (2018). Delays to treatment initiation is associated with tuberculosis treatment outcomes among patients on directly observed treatment short course in Southwest Ethiopia: A follow-up study. BMC Pulmonary Medicine, 18(1), 64. 10.1186/s12890-018-0628-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awoke N, Dulo B, & Wudneh F (2019). Total Delay in Treatment of Tuberculosis and Associated Factors among New Pulmonary TB Patients in Selected Health Facilities of Gedeo Zone, Southern Ethiopia, 2017/18. Interdisciplinary Perspectives on Infectious Diseases, 2019. 10.1155/2019/2154240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojovic O, Medenica M, Zivkovic D, Rakocevic B, Trajkovic G, Kisic-Tepavcevic D, & Grgurevic A (2018). Factors associated with patient and health system delays in diagnosis and treatment of tuberculosis in Montenegro, 2015–2016. PLOS ONE, 13(3), e0193997. 10.1371/journal.pone.0193997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A (2005). Mode of questionnaire administration can have serious effects on data quality. Journal of Public Health, 27(3), 281–291. 10.1093/pubmed/fdi031 [DOI] [PubMed] [Google Scholar]
- Coimbra I, Maruza M, Militão-Albuquerque M. de F. P., Moura LV, Diniz GTN, Miranda-Filho D. de B., Lacerda HR, Rodrigues LC, & Ximenes R. A. de A. (2012). Associated factors for treatment delay in pulmonary tuberculosis in HIV-infected individuals: a nested case-control study. BMC Infectious Diseases, 12, 208. 10.1186/1471-2334-12-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Basu M, Mandal A, Roy N, Chatterjee S, & Dasgupta A (2017). Prevalence and determinants of delay in diagnosis of pulmonary tuberculosis in Darjeeling district of West Bengal. Journal of Family Medicine and Primary Care, 6(3), 627. 10.4103/2249-4863.214432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasa TT, Roba AA, Weldegebreal F, Mesfin F, Asfaw A, Mitiku H, Teklemariam Z, Geddugol BJ, Naganuri M, Befikadu H, & Tesfaye E (2019). Prevalence and associated factors of depression among tuberculosis patients in Eastern Ethiopia. BMC Psychiatry, 19(1), 82. 10.1186/s12888-019-2042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AM, Kelly J, McDonald C, O’Dywer AM, Keane J, & Cooney J (2013). A review of the interplay between tuberculosis and mental health. General Hospital Psychiatry, 35(4), 398–406. 10.1016/j.genhosppsych.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, & Altman DG (2016). Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. European Journal of Epidemiology, 31(4), 337–350. 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ige OM, & Lasebikan VO (2011). Prevalence of depression in tuberculosis patients in comparison with non-tuberculosis family contacts visiting the DOTS clinic in a Nigerian tertiary care hospital and its correlation with disease pattern. Mental Health in Family Medicine, 8(4), 235–241. [PMC free article] [PubMed] [Google Scholar]
- Janmeja AK, Das SK, Bhargava R, & Chavan BS (2005). Psychotherapy Improves Compliance with Tuberculosis Treatment. Respiration, 72(4), 375–380. 10.1159/000086251 [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg A, Dube A, Curran R, Ambaw F, Murdoch J, Bachmann M, Petersen I, & Fairall L (2020). Comorbidities between tuberculosis and common mental disorders: A scoping review of epidemiological patterns and person-centred care interventions from low-to-middle income and BRICS countries. In Infectious Diseases of Poverty (Vol. 9, Issue 1, pp. 1–18). BioMed Central Ltd. 10.1186/s40249-019-0619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehbila J, Ekabe CJ, Aminde LN, Noubiap JJN, Fon PN, & Monekosso GL (2016). Prevalence and correlates of depressive symptoms in adult patients with pulmonary tuberculosis in the Southwest Region of Cameroon. Infectious Diseases of Poverty, 5(1), 51. 10.1186/s40249-016-0145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, & Glaser R (2002). Depression and immune function Central pathways to morbidity and mortality. Journal of Psychosomatic Research, 53(4), 873–876. 10.1016/s0022-3999(02)00309-4 [DOI] [PubMed] [Google Scholar]
- Koyanagi A, Vancampfort D, Carvalho AF, DeVylder JE, Haro JM, Pizzol D, Veronese N, & Stubbs B (2017). Depression comorbid with tuberculosis and its impact on health status: cross-sectional analysis of community-based data from 48 low- and middle-income countries. BMC Medicine, 15(1), 209. 10.1186/s12916-017-0975-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ehiri J, Tang S, Li D, Bian Y, Lin H, Marshall C, & Cao J (2013). Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Medicine, 11(1), 156. 10.1186/1741-7015-11-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Wellness. (2018). Botswana National TB Program. [Google Scholar]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, … Lopez AD (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2197–2223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- Nogueira BMF, Rolla VC, Akrami KM, & Kiene SM (2018). Factors associated with tuberculosis treatment delay in patients co-infected with HIV in a high prevalence area in Brazil. PLoS ONE, 13(4). 10.1371/journal.pone.0195409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KH, Choi H, Kim EJ, Kim HJ, & Cho SI (2017). Depression & risk of tuberculosis: A nationwide populationbased cohort study. International Journal of Tuberculosis and Lung Disease, 21(7), 804–809. 10.5588/ijtld.17.0038 [DOI] [PubMed] [Google Scholar]
- Pachi A, Bratis D, Moussas G, & Tselebis A (2013). Psychiatric Morbidity and Other Factors Affecting Treatment Adherence in Pulmonary Tuberculosis Patients. Tuberculosis Research and Treatment, 2013, 1–37. 10.1155/2013/489865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, Chisholm D, Collins PY, Cooper JL, Eaton J, Herrman H, Herzallah MM, Huang Y, Jordans MJD, Kleinman A, Medina-Mora ME, Morgan E, Niaz U, Omigbodun O, … UnÜtzer Jü. (2018). The Lancet Commission on global mental health and sustainable development. The Lancet, 392(10157), 1553–1598. 10.1016/S0140-6736(18)31612-X [DOI] [PubMed] [Google Scholar]
- Segagni Lusignani L, Quaglio G, Atzori A, Nsuka J, Grainger R, Da Conceiçao Palma M, Putoto G, & Manenti F (2013). Factors associated with patient and health care system delay in diagnosis for tuberculosis in the province of Luanda, Angola. BMC Infectious Diseases, 13(1), 168. 10.1186/1471-2334-13-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botswana Statistics. (2015). Botswana Population Projections 2011–2026. http://www.statsbots.org.bw/sites/default/files/publications/population_projection.pdf [Google Scholar]
- Steen TW, & Mazonde GN (1998). Pulmonary tuberculosis in Kweneng District, Botswana: Delays in diagnosis in 212 smear-positive patients. In International Journal of Tuberculosis and Lung Disease (Vol. 2, Issue 8, pp. 627–634). [PubMed] [Google Scholar]
- Storla DG, Yimer S, & Bjune GA (2008). A systematic review of delay in the diagnosis and treatment of tuberculosis. In BMC Public Health (Vol. 8, p. 15). BioMed Central Ltd. 10.1186/1471-2458-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetland AC, Kritski A, Oquendo MA, Sublette ME, Pala AN, Silva LRB, Karpati A, Silva EC, Moraes MO, Silva JRLE, & Wainberg ML (2017). Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. International Journal of Tuberculosis and Lung Disease, 21(8), 852–861. 10.5588/ijtld.16.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarinda KC, Harries AD, Nyathi B, Ngwenya M, Mutasa-Apollo T, & Sandy C (2015). Tuberculosis treatment delays and associated factors within the Zimbabwe national tuberculosis programme. BMC Public Health, 15(1), 29. 10.1186/s12889-015-1437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita A, Ramlall S, Naidu T, Mthembu SS, Padayatchi N, & Burns JK (2019). Major depression and household food insecurity among individuals with multidrug-resistant tuberculosis (MDR-TB) in South Africa. Social Psychiatry and Psychiatric Epidemiology, 54(3), 387–393. 10.1007/s00127-019-01669-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, & Greer TL (2014). Cognitive dysfunction in unipolar depression: Implications for treatment. In Journal of Affective Disorders (Vols. 152–154, Issue 1, pp. 19–27). 10.1016/j.jad.2013.09.012 [DOI] [PubMed] [Google Scholar]
- van den Heuvel L, Chishinga N, Kinyanda E, Weiss H, Patel V, Ayles H, Harvey J, Cloete KJ, & Seedat S (2013). Frequency and correlates of anxiety and mood disorders among TB- and HIV-infected Zambians. AIDS Care, 25(12), 1527–1535. 10.1080/09540121.2013.793263 [DOI] [PubMed] [Google Scholar]
- Wang XB, Li XL, Zhang Q, Zhang J, Chen HY, Xu WY, Fu YH, Wang QY, Kang J, & Hou G (2018). A survey of anxiety and depressive symptoms in pulmonary tuberculosis patients with and without tracheobronchial tuberculosis. Frontiers in Psychiatry, 9(JUL). 10.3389/fpsyt.2018.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2006). Diagnostic and treatment delay in tuberculosis. http://applications.emro.who.int/dsaf/dsa710.pdf.
- Wondawek TM, & Ali MM (2019). Delay in treatment seeking and associated factors among suspected pulmonary tuberculosis patients in public health facilities of Adama town, eastern Ethiopia. BMC Public Health, 19(1), 1527. 10.1186/s12889-019-7886-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. (2019a). Botswana Tuberculosis Profile. Global Tuberculosis Report. https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=BW&outtype=html [Google Scholar]
- World Health Organisation. (2019b). Global Tuberculosis Report. [Google Scholar]
- Xavier PB, & Peixoto B (2015). Emotional distress in angolan patients with several types of tuberculosis. African Health Sciences, 15(2), 378–384. 10.4314/ahs.v15i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G (2004). Modified Poisson Regression Approach to Prospective Studies with Binary Data | American Journal of Epidemiology | Oxford Academic. American Journal of Epidemiology, 159(7), 702–706. https://academic.oup.com/aje/article/159/7/702/71883 [DOI] [PubMed] [Google Scholar]
- Zung WWK (1971). A Rating Instrument For Anxiety Disorders. Psychosomatics, 12(6), 371–379. 10.1016/S0033-3182(71)71479-0 [DOI] [PubMed] [Google Scholar]


