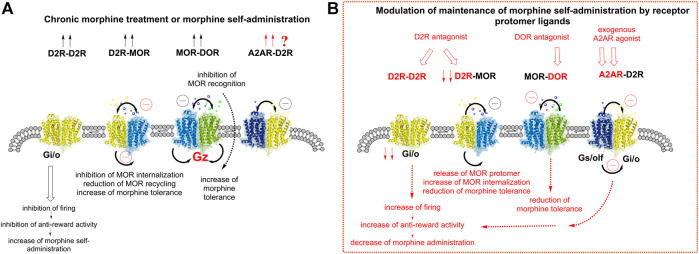
FIGURE 2.
Understanding the role of the A2AR-D2R heteroreceptor complexes in modulating the changes in the activity of the ventral striatal-pallidal GABA antireward neurons induced by chronic morphine treatment or morphine self-administration. (A) Morphine (chronic) is shown to increase the density of D2R-D2R homomers (2 arrows pointing upwards). This causes enhanced inhibition of firing in the antireward neurons leading to a reduction of antireward activity. An increase of morphine self-administration is found. The D2R-MOR complex also becomes increased in density after morphine (chronic) mainly due to inhibition MOR internalization. This event will result in a reduction of MOR cycling, which impairs its signaling, and a reduction of recognition also develops due to antagonistic allosteric receptor-receptor interactions. As a result, an increase in morphine tolerance takes place since higher doses of morphine are needed to induce reward due to the malfunction of the MOR signaling. It is also proposed that an increased density of A2AR-D2R complexes develops upon exposure to chronic morphine as seen from the two red arrows indicated. As a result, the D2R function becomes reduced through an allosteric brake on D2R signaling. The MOR-DOR complex is well known to be increased in density upon chronic morphine treatment or morphine self-administration, shown by 2 arrows. Both receptor protomers remain functional by coupling to Gz protein. However, the morphine activation of the DOR protomer is known to produce a brake on MOR recognition via an allosteric receptor-receptor interaction. Therefore, an increase in morphine tolerance takes place. (B) Modulation of morphine effects by receptor protomer ligands. The D2R antagonist is known to block the inhibitory D2R homomer signaling over Gi/o and produces a marked reduction of morphine self-administration. In the case of the D2R-MOR complex, the D2R antagonist appears to disrupt the complex and set the MOR protomer free from D2R mediated allosteric inhibition. MOR internalization is therefore increased and its function restored, leading to a reduction of morphine tolerance due to enhanced MOR signaling induced by morphine. It is indicated that the A2AR agonist given in vivo should effectively inhibit the function of the D2R protomer of the A2AR-D2R complex, increased in density (see A). Thus, a reduction of the inhibitory Gi/o activity of the D2R protomer develops which results in an increase in the activity of the anti-reward GABA neurons. A reduction of morphine self-administration should develop. The DOR antagonist can target the DOR protomer and remove its allosteric inhibition of the MOR signaling, which reduces morphine tolerance.