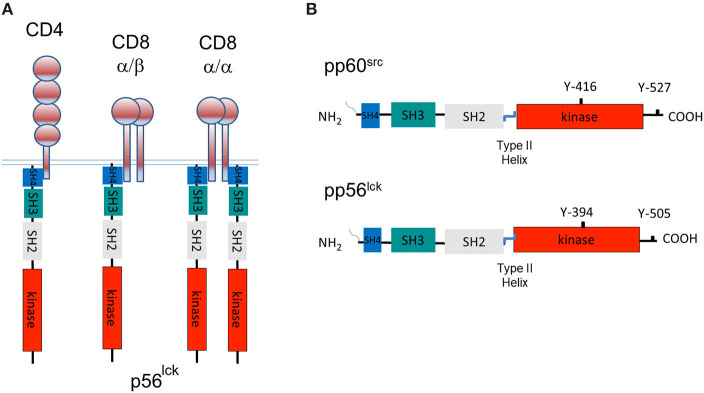
Figure 1.
A tale of three CD4 and CD8-p56lck complexes and the structure of pp60src and p56lck. (A) The model of three CD4 and CD8-p56lck complexes in T-cells. CD8 is expressed as a CD8α homodimer as well as a CD8α/β heterodimer. p56lck binds to the α subunit but not the β subunit. CD8α homodimer has two p56lck bound molecules and the CD8α/β heterodimer has a single p56lck bound. CD4 binds to p56lck in a monomeric form. (B) Structure of pp60src and p56lck. p56lck is an immune cell-enriched member of the pp60src family of protein-tyrosine kinases. p56lck is myristoylated and palmitoylated at the N-terminus, while Src lacks palmitoylation sites. This region is followed by poorly conserved unique SH4 region which in the case of p56lck binds to the cytoplasmic tails of CD4 and CD8, an SH3 domain that binds to proline-rich residues, an SH2 domain that binds to specific sites that are tyrosine phosphorylated, an SH2-kinase linker region, an SH1 kinase domain followed by a C-terminal negative regulatory region. The C-terminal tail has an inhibitory Y-527 site when phosphorylated, in the case of pp60src and a Y-505 site in p56lck. pp60src and p56lck also possess an auto-phosphorylation site in the kinase domain of each kinase corresponding to Y-416 in the case of pp60src and Y-394 in the case of p56lck.