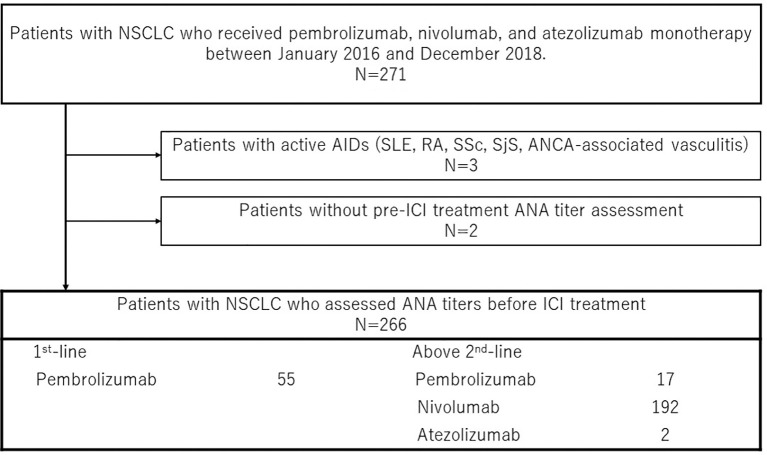
Figure 1.
Study schema, antinuclear antibody (ANA) titers in 271 patients with advanced NSCLC were assessed before anti-PD-1/PD-L1 antibodies monotherapy administration at Saitama Medical University, International Medicine Center. Patients treated previously with other ICIs (Pembrolizumab and nivolumab including 1st-line therapy) or who did not have pre-treatment ANA titers before ICI or who had active major AIDs were excluded. Nivolumab, pembrolizumab, and atezolizumab were administered to 192, 72 (55 as 1st setting), and 2 patients. PFS, progression-free survival; OS, overall survival; ICI, immune checkpoint inhibitors; PD-1, programmed death-1; PD-L1, programmed death ligand 1; ANA, antinuclear antibody.