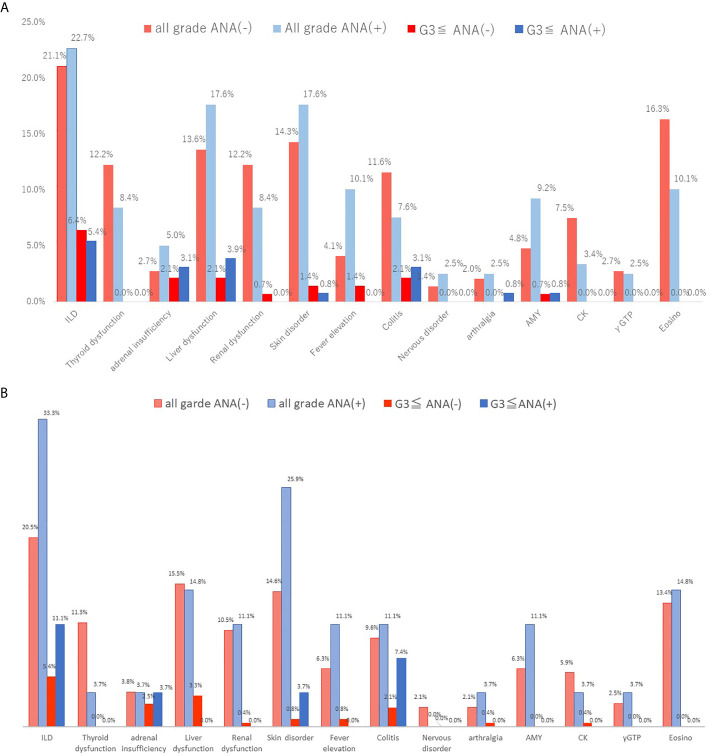
Figure 3.
Immune-related adverse events between ANA-positive and ANA-negative patients (cutoff of 1:40) or high and low ANA (cutoff of 1:80). The irAEs were listed at frequency 2% or more in either group. (A) When a positive ANA cutoff of 1:40 was used, (B) when a positive ANA cutoff of 1:80 was used. irAEs, immune-related adverse events; AMY, amylase; CK, creatine kinase; γGTP, γ- Glutamyltranspeptidase.