Figure 1.
Optimization of electroporation conditions for NKG2D CAR-T cell generation
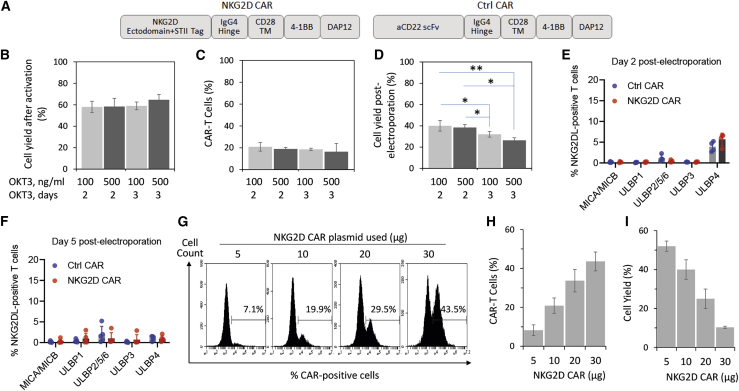
(A) NKG2D CAR and control CAR constructs used in the current study. (B) PBMC activation. Donor-derived PBMCs were activated with either 100 ng/mL or 500 ng/mL of OKT3 for either 2 or 3 days. Cell yield was measured with trypan blue exclusion assay after the activation and is presented as a percentage of total PBMCs initially used. (C) Electroporation efficiency. Activated PBMCs were transfected by electroporation of 5 μg of piggyBac transposase plasmid and 10 μg of NKG2D CAR donor plasmid in trial runs to evaluate cell survival. The percentage of CAR-T cells was measured by flow cytometry with an antibody against Streptavidin II (STII) tag on day 5 post-electroporation. (D) Cell yield post-electroporation. Electroporation was performed as described in (C). Cell yield as a percentage of total activated T cells electroporated was measured with trypan blue exclusion assay on day 5 post-electroporation. (E and F) Expression profile of NKG2D ligands on T cells at 2 days (E) and 5 days (F) post-electroporation. Cells were analyzed through staining with individual antibodies against MICA/MICB, ULBP1, ULBP2/5/6, ULBP3, and ULBP4. (G–I) Dose-dependent expression of NKG2D CAR on T cells 5 days post-electroporation. (G) Representative flow cytometry histograms from a single donor electroporated with 5 μg of piggyBac transposase plasmid and 5, 10, 20, or 30 μg of NKG2D CAR donor plasmid. (H) Electroporation efficiency increases with increasing amounts of the CAR plasmid used. (I) Cell yield as a percentage of total activated T cells electroporated decreases with increasing amounts of the CAR plasmid used. All data in (B)–(D), (H), and (I) are mean ± SD of three independent experiments with PBMC samples from three different donors. Data in (E) and (F) are mean ± SD of single measurements from five different donors.