Figure 4.
Cryopreservation to facilitate multiple injections of NKG2D CAR-T cells
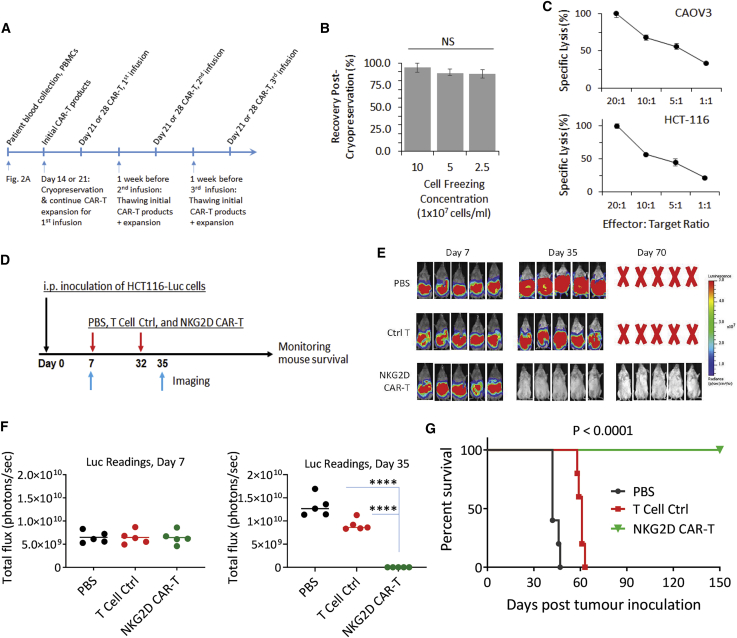
(A) Schematic timeline to illustrate time points where cryopreservation and thawing of NKG2D CAR-T cells are performed. Cryopreservation on either day 14 or day 21 and subsequent thawing and expansion 1 week before infusion. A possible clinical application timeline with 3 infusions separated by 2 weeks is shown. (B) Varying freezing densities did not affect cell recovery yields of cryopreserved day 21 NKG2D CAR-T cells. CAR-T cells, after freezing for 2 weeks, were thawed and enumerated with a trypan blue exclusion assay immediately after thawing. Duplicate readings from each single donor were obtained from 2 independent thaw/count procedures. Average values were then pooled from 3 independent donors to derive mean ± SD shown in the chart. (C) Cryopreservation and subsequent K562-based expansion did not compromise antigen-dependent cytotoxicity against both CAOV3 and HCT-116 target cell lines. Cytotoxicity assay was performed at effector-to-target ratios of 20:1, 10:1, 5:1, and 1:1 in a standard DELFIA time-resolved fluorescence assay. Data shown are mean ± SD from a single representative assay. (D) Experimental outline of an animal study using cryopreserved NKG2D CAR-T cells. Three groups of mice (5 mice per group) received i.p. injection of 2 × 106 HCT116-Luc cells (day 0) followed by i.p. injection of PBS, ctrl T cells, or NKG2D CAR-T cells on day 7 and day 32 (1 × 107 cells per mouse). Bioluminescent imaging of tumor signals was performed on day 7 and day 35. (E) Bioluminescent images on day 7 and day 35 are shown. (F) Bioluminescence flux values on day 7 and day 35. The values from each mice of respective groups are plotted. ∗∗∗∗p < 0.0001. (G) Kaplan-Meier analysis of survival of the in vivo animal experiment. Statistical analysis between groups was performed with the log-rank test.