Abstract
Ochratoxin A (OTA) is a widespread mycotoxin, that has strong thermal stability, and is difficult to remove from feed. OTA is nephrotoxic, hepatotoxic, teratogenic, immunotoxic, and enterotoxic to several species of animals. The gut is the first defense barrier against various types of mycotoxins present in feed that enter the body, and it is closely connected to other tissues through enterohepatic circulation. Compared with mammals, poultry is more sensitive to OTA and has a lower absorption rate. Therefore, the gut is an important target tissue for OTA in poultry. This review comprehensively discusses the role of OTA in gut health and the gut microbiota of poultry, focusing on the effect of OTA on digestive and absorptive processes, intestinal barrier integrity, intestinal histomorphology, gut immunity, and gut microbiota. According to the studies described to date, OTA can affect gut dysbiosis, including increasing gut permeability, immunity, and bacterial translocation, and can eventually lead to gut and other organ injury. Although there are many studies investigating the effects of OTA on the gut health of poultry, further studies are needed to better characterize the underlying mechanisms of action and develop preventative or therapeutic interventions for mycotoxicosis in poultry.
Key words: ochratoxin A, poultry, tight junction, immunity, gut microbiota
Graphical abstract
Introduction
Mycotoxins, which have a low molecular mass (∼ 700 Da), are secondary metabolites produced by fungal genera (e.g., Aspergillus, Penicillium, and Fusarium) and are the most common natural food contaminants in components of human and animal diets, such as cereals and animal forages (Wild and Gong, 2010; Manizan et al., 2018). Fungal proliferation and production of mycotoxins arise naturally due to environmental factors, such as tropical conditions (Mohd-Redzwan et al., 2013), the warming earth (Medina et al., 2015), and downstream processing (Khazaeli et al., 2014). According to a survey, 25% of the world's crops, such as nuts, grains, and rice, are contaminated by molds and fungi (Pandya and Arade, 2016). The detection rates of aflatoxin B1, deoxynivalenol, zearalenone, and fumonisin in layer feed were 83.16, 93.68, 94.74, and 100%, respectively, and those in meat-type poultry feed were 89, 96, 85, and 94.74% in Southwest China (Li, 2016). Due to the ubiquitous nature of fungi, mycotoxins have been increasingly attracting the concern of health organizations where their occurrence in foods cannot be ignored and already poses a risk to consumers (Jahanian, 2016). When animals consume mycotoxin-contaminated diets, intestinal epithelial cells (IECs) are completely exposed to the contaminated feed, the epithelial cells of the esophagus and gastrointestinal tract (GIT) can be damaged, easily leading to gastrointestinal dysfunction, intestinal microbial imbalance, malnutrition, diarrhea, vomiting, and intestinal inflammation, and ultimately decreased growth performance (Zhang, 2018; Ruan et al., 2019; Wang et al., 2019).
Ochratoxins are a group of secondary metabolites produced by fungi of 2 genera: Penicillium and Aspergillus (Ringot et al., 2006). Ochratoxin A (OTA) is the most common and toxic ochratoxin (Liuzzi et al., 2016). It has been shown to be nephrotoxic, hepatotoxic, teratogenic, and immunotoxic to poultry (Hassan et al., 2012b; Hameed et al., 2017; Bhatti et al., 2019; Wang et al., 2019). Mycotoxin uptake and subsequent tissue distribution are mediated by the GIT. When mycotoxins are introduced into organisms via food or feed, they first interact with the GIT (Assunção et al., 2016). There is an increasing awareness of the deleterious effects of mycotoxins within the GIT. A healthy intestinal tract guarantees the welfare and health of both people and animals. Rapidly dividing and activated cells and tissues with a high protein turnover are predominant in the gut epithelium. Intestinal cells and tissues could become a main target of mycotoxins as many of these metabolites are inhibitors of protein synthesis. The absorption of OTA starts from the stomach and mainly occurs in the proximal jejunum (Grenier and Applegate, 2013). The absorption rate of OTA was low in poultry (Galtier et al., 1981), and the unabsorbed OTA could reach the hindgut to interact with the intestinal microbiota. Considering this background, the current review aims to provide a summary of the available evidence regarding the effects of OTA on intestinal health and microbiota in poultry.
OTA
Ochratoxin is mainly produced by Aspergillus and Penicillium. OTA (C20H18ClNO6; molecular weight: 403.8) is a white, tasteless, and heat-stable crystalline solid preparation (melting point, 168°C–173°C) with poor water solubility (Pohland et al., 1982). OTA does not completely disappear during baking (Vidal et al., 2015); furthermore, OTA can resist 3 h of autoclaving at 121°C (Trivedi et al., 1992), and even at 250°C, it is only partially degraded (Boudra et al., 1995). During coffee roasting, OTA is only partially decomposed (Blanc et al., 1998; Gerrit et al., 2001). A structural analysis of OTA showed that OTA is a secondary metabolite derived from polyketide, which contains a dihydrocoumarin moiety coupled to a l-β-phenylalanine through an amide bond. The metabolite is derived from the shikimic acid pathway. The specific structure of OTA is shown in Figure 1 (André and Ali, 2010). OTA is a weak acid and has a strong capacity to bind with proteins. Once it enters the blood stream, approximately 99% of OTA binds to serum albumin. This binding contributes to the passive absorption of OTA in the nonionic form (Chu, 1971, 1974; Galtier et al., 1981; Hagelberg et al., 2010). After conjugated OTA reaches the hindgut, it is released by intestinal microorganisms, and a second peak appears in the intestinal contents, which is absorbed by the intestine again (Kumagai et al., 1985; Plestina, 1988; Roth et al., 1988; Sreemannarayana et al., 1988). Therefore, OTA may affect the intestinal microbiota, and OTA toxicity in the intestinal tract may be stronger than that in other tissues.
Figure 1.
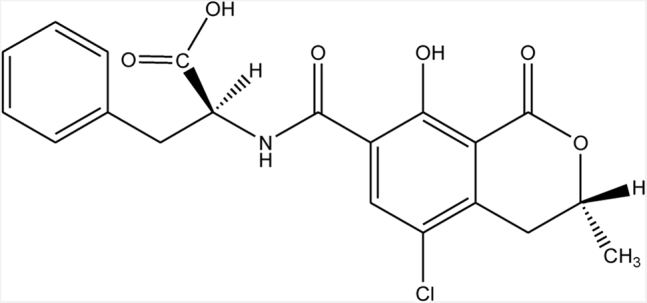
Chemical structure of ochratoxin A (André and Ali, 2010).
Prevalence of OTA
As one of the most common toxins in food or feed, OTA is widely found in cereals (Mario et al., 2009), coffee, wine, dried fruits, nuts (Fink-Gremmels, 2005; Gagliano et al., 2006), herbal medicines (tangerine seeds, 92.3 μg/kg; Trichosanthis Semen, 6.05 ng/g; milk thistle, 10 μg/kg (Shim et al., 2014; Veprikova et al., 2015; Chen et al., 2015), food coloring agents (1.16–32.00 μg/kg), plant food supplements (1.16–20.03 μg/kg) (Solfrizzo et al., 2015), bottled water (0.2 ng/L) (Mata et al., 2015), and fresh sweet peppers (29.5 μg/kg) (Gambacorta et al., 2018). Among 87 human milk samples, 84 (96.6%) samples were positive for OTA at a mean level of 24.57 ± 13.6 ng/L, and 14 (16%) were positive samples with the maximum limit of 40 ng/L for ochratoxin (Parvin et al., 2014). An investigation showed that the probability of OTA detection in fresh sweet peppers was 51%, and the average content of OTA was 29.5 μg/kg (Gambacorta et al., 2018). The wide occurrence of OTA and its high thermal stability make the eradication of OTA from the food chain very difficult (Frantisek et al., 2016). OTA is also found in feed and feedstuffs. A study showed that OTA in mixed feed and feedstuffs used in some laying hens was found to be positive in 64 of the 89 samples analyzed (Gumus et al., 2018). In another study from South America published by Magnoli et al. (2007), OTA was present at a mean level of 25 to 30 μg/kg in poultry feedstuffs. Mycotoxins may remain in the tissues and eggs of poultry-fed moldy feed. Among 115 chicken meat and 80 egg samples, 41% of meat samples (average content of OTA was 1.41 ± 0.70 μg/kg), and 35% of the egg samples were positive for OTA (average content of OTA was 1.17 ± 0.42 μg/kg) (Iqbal et al., 2014), and the residues of OTA in eggs consistently increased with time and reached a maximum (7.396 ± 1.03 ng/g) at day 21 of toxin feeding with 5 mg of OTA/kg of feed (Hassan et al., 2012a). The OTA residue in eggs may harm the offspring, which was confirmed by Hassan et al. (2012b). After 2 wk of OTA administration in ducks (235 μg/kg body weight), the OTA residue concentrations in the kidney, cecum, liver, pectoral muscle, duodenum, jejunum, ileum, and leg muscle were 1.59, 0.94, 0.92, 0.54, 0.42, 0.41, 0.32, and 0.20 ng/kg, respectively (Wang et al., 2019). According to the these results, OTA residues were quite high in the intestine, especially in the cecum. These findings partly explain the enterotoxicity of OTA.
Limitation of OTA in Feed
Due to its toxic properties, OTA is subject to legal regulation at both the national and international level. However, to date, only a nonbinding recommendation exists for OTA levels in cereal feed and feed for poultry in the European Union (Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of OTA in products intended for animal feeding, see Table 1) (European Union, 2006), and the limit for OTA in poultry feed is 0.10 mg/kg.
Table 1.
Guidance values for ochratoxin A under Commission Recommendation 2006/576/EC as in force.
| Feed | Guidance value in mg/kg relative to feedstuffs with a moisture content of 12% |
|---|---|
| Feed materials—cereals and cereal products Complementary and complete feedstuffs |
0.25 |
| Complementary and complete feedstuffs for pigs | 0.05 |
| Complementary and complete feedstuffs for poultry | 0.10 |
Sensitivity of Animals to OTA
Different animals have different sensitivities to OTA. Normally, young animals are more sensitive to OTA than mature ones due to the incomplete development of organs (Sherif et al., 2009; Adetunji et al., 2017). A study has shown that when 1-day-old ducks were fed OTA for 6 d, the median lethal dose (LD50) of the ducks was 0.5 mg/kg body weight (Van Der Merwe et al., 1965), while when 1-day-old Babcock B-300 cockerels were fed OTA for 7 d, the LD50 was 3.3 mg/kg body weight (Peckham et al., 1971). The comparative acute and oral toxicity of OTA for 3-day-old avian species is presented. The 7-day LD50 values for White Leghorns, turkeys, and Japanese quail were 3.4 ± 0.19, 5.9 ± 0.72, and 16.5 ± 0.56 mg/kg body weight, respectively (Prior et al., 1976). The LD50 for rats was 22 mg/kg for males and 20 mg/kg for females (Purchase et al., 1968). From these results, compared with mammals, poultry are considered to be more sensitive to OTA. Therefore, researchers should determine the effect of OTA on poultry health, especially on intestinal health, and pay attention to the contamination of OTA in poultry feed and feedstuff.
GIT
The GIT is responsible for food ingestion, digestion, energy and nutrient absorption, immune responses, and elimination of waste products (feces) (Pietro et al., 2017). GIT is a muscular tube forming a continuous passage that is lined by a mucous membrane consisting of 4 layers. All segments of the GIT are divided into 4 layers: the mucosa (epithelium, lamina propria, and muscular mucosa), the submucosa, the muscularis propria (inner circular muscle layer, intermuscular space, and outer longitudinal muscle layer), and the serosa (Jaladanki and Wang, 2016). The entire mucosa rests on the submucosa, beneath which is the muscularis propria. The outermost layer is named the serosa. The complex infoldings at the mucosal layer form an immense surface area for most efficient nutrient absorption. The submucosa contains arteries, veins, inflammatory cells, lymphatics, autonomic nerve fibers, and ganglion cells. The muscularis mucosa is a thin layer of smooth muscle that forms the basis of the peristalsis. The serosa is made of connective tissue that contains blood vessels, nerves, and fat. The innermost epithelial layer of the mucosa is of vital importance for intestinal barrier function. The intestinal epithelium is one layer of thin cells lining the gut lumen. The epithelium contains enterocytes, enteroendocrine, and goblet cells at villi, whereas Paneth cells are located under crypts (Fink et al., 2016). The epithelium serves as a barrier to block the entry of harmful agents such as pathogens, toxins, and foreign antigens. In addition, it plays an important role in nutrient absorption, including that of electrolytes, dietary nutrients, and water via its selective permeable membrane (Constantinescu and Chou, 2016). Each IEC is connected by desmosomes, tight junctions (TJs), and adherent junctions. The adherent junctions and desmosomes are responsible for the mechanical linkage of adjacent cells. TJs control the intercellular space and regulate selective paracellular ionic solute transport (Capaldo et al., 2014).
Modulation of Digestive and Absorptive Processes by OTA
The absorption of OTA starts from the stomach, and occurs mainly in the proximal jejunum. It enters IECs by passive diffusion, mainly by nonionic absorption, and enters the blood circulation to reach all tissues and organs of the body (Grenier and Applegate, 2013). The effect of mycotoxins on nutrient and energy digestibility has been documented. OTA in combination with aflatoxin had a more pronounced effect on the metabolizable energy content of the diet than the toxin in feed alone, and this reduction occurred through a significant increase in the maintenance energy requirement of hens (Verma et al., 2007). Razafimanjato et al. (2010) found that OTA inhibited the absorption of glutamate by decreasing the expression of glutamate/aspartate transporter and glutamate transporter at the cell surface. Feeding meat ducks with OTA-contaminated diets decreased the content of alanine, leucine, and glutamate in plasma, downregulated the expression of sodium-dependent glutamate transporter 1 mRNA in the jejunum, and decreased the mRNA expression of cationic amino acid transporter 1, excitatory amino acid transporter 4, and peptide transporter (Ruan, 2018). These results indicated the necessity for considering not only the OTA contamination in evaluating their effect on poultry health and performance, but also the possible physicochemical alterations of feedstuffs due to the OTA contamination. More studies should focus on the effect of OTA on nutrient digestibility and nutrient requirements of animals under mycotoxin contamination.
OTA Destroys Intestinal Barrier Integrity
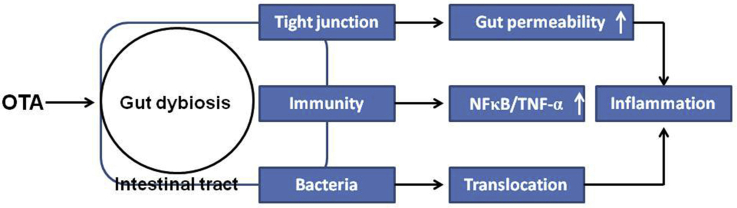
IECs have 2 crucial but conflicting functions. On one hand, they transport nutrients and fluids; on the other hand, they restrict the access of luminal antigens to the internal milieu. They form a monolayer that constitutes a dynamic and selective barrier and mediates the transport of molecules in 2 ways: either across the cells (i.e., the transcellular pathway) or between the cells (i.e., the paracellular pathway) (Grenier and Applegate, 2013). When OTA are introduced into organisms from food, they first interact with the GIT. The TJs in the intestinal epithelium are important barriers for maintaining intestinal epithelial permeability, which is greatly assisted by TJ proteins that prevent harmful substances from entering the body and maintain the structural integrity of TJs (Buckley and Turner, 2018). Many studies have shown that OTA can destroy intestinal barrier integrity by suppressing TJ proteins, such as occludin, zonula occludens-1, and claudin-1, in poultry (Ruan et al., 2019; Wang et al., 2019; Yang et al., 2020). The increase in intestinal permeability, in turn, promotes the translocation of bacteria (Kelly et al., 2015). Together, OTA could destroy TJs, such as downregulating the expression of TJ genes and proteins and increasing the serum lipopolysaccharide content, thus increasing the gut permeability. The increased gut permeability may subsequently enable the translocation of harmful stressors, particular bacteria (commensals and pathogens), from the gut lumen to the internal environment and eventually cause harm to animals.
OTA Damages the Intestinal Histomorphology
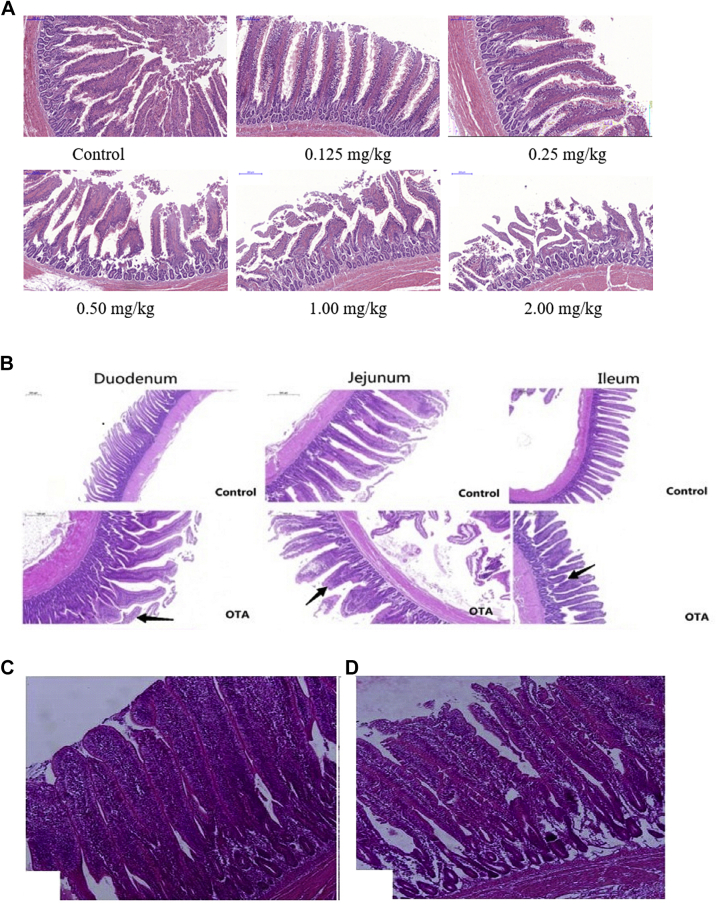
The intestine is the largest mucosal tissue, and plays an important role in immune homeostasis. Mucus secretion by goblet cells is significant for the maintenance of mucosal homeostasis, which helps to maintain intestinal homeostasis (Johansson and Hansson, 2016; McGuckin and Hasnain, 2017). Although the kidney is the main target for OTA-induced toxicity, histological abnormalities and lesions of the GIT have been reported. In chickens, a decrease in villus height was observed in combination with an increase in necrosis and apoptosis of IECs with prismatic epithelium and alterations of brush border (Solcan et al., 2015). Ducks fed a diet containing 2 mg/kg OTA showed villous blunting and epithelial denudation along with a related decrease in villous height and increase in the depth of crypts (Figure 2A, Ruan, 2018). Similar studies have confirmed this result by Tong et al. (2020) (Figure 2B) and Malekinezhad et al. (2020) (Figure 2C). They reported that OTA decreased the villus height/crypt depth ratio and goblet cells of duodenum, jejunum, and ileum, and the cecum was inflated and bleeding, and a small amount of intestinal mucosa was detached in broilers. Alteration of intestinal histomorphology could reduce nutrient absorption, and the decrease in goblet cells rendered the gut vulnerable to infection. OTA exerts its effect on the gut via reduction of nutrient absorption, disruption of intestinal permeability, and modulation of the immune system.
Figure 2.
OTA damages the intestinal histomorphology. (A) Ducks fed diets with different doses of OTA showed villous blunting and epithelial denudation in the jejunum, compared with the controls (magnification, 200×; scale bar, 200 μm; Ruan, 2018). (B) Detection of pathological changes in duodenum, jejunum, and ileum tissue (magnification, 5×; scale bar, 500 μm). The arrows “→” indicate a intestinal pathological damage or breakage of broiler chickens received 50 µg of OTA/kg body weight/day for 21 d (Tong et al., 2020). (C) Histological structures of the ileum at 42 d of age (magnification, 5×): negative control diet with normal epithelial cells in the apical region of villi; (D) diet contaminated with OTA; the villus height was decreased and the apical region of epithelial cells was shedding (Malekinezhad et al., 2020). Abbreviation: OTA, ochratoxin A.
OTA and Gut Immunity
The first expression of OTA toxicity is immunosuppression, which may become clinically apparent before nephropathy is manifested (Aleo et al., 1991). The gut is the first defense barrier against various types of contamination in food (Grenier and Oswald, 2011); therefore, compared with other tissues, it is exposed to mycotoxins at higher concentrations upon ingestion of contaminated food (Marie-Caroline et al., 2016). Furthermore, immune cells of the gut-associated lymphoid tissue are more numerous than those of other secondary lymphoid tissues contained in the whole body, and the intestine plays an important role in immunity in the host (Castro and Arntzen, 1993). Seven-day-old male broiler chickens (Ross 308) were administered 20 and 50 μg/kg body weight OTA by gavage. After 14 d of exposure, OTA reduced the lymphocyte population in the intestinal epithelium and the lamina propria, and increased the CD4+ and CD8+ values in both the duodenum and jejunum after 28 d of administration (Solcan et al., 2015). OTA is a fungal metabolite with immunomodulatory effects. A study has suggested that OTA induces the secretion of proinflammatory cytokines, such as IL-1β and tumor necrosis factor α, in the jejunum of Pekin ducklings; moreover, OTA reduces the production of the anti-inflammatory factor IL-10 and decreases the IgA content in the jejunum (Ruan, 2018). A similar result was confirmed by Tong et al. (2020), who demonstrated that 1-day-old broiler chickens fed 50 μg of OTA/kg body weight show upregulated mRNA expression of IL-1β and tumor necrosis factor α and induced the phosphorylation of nuclear factor κB. The OTA group had a severely congested lamina propria of the intestine, in filtrated lymphocytes; several necrotic epithelial cells; and the intestinal villi were short, thick, and edematous at various degrees. The effect of OTA on gut immunity is summarized in Table 2. These results suggested that OTA is cytotoxic to the intestinal epithelium and mucosa-associated lymphoid tissue, altering the intestinal barrier and increasing susceptibility to various associated diseases.
Table 2.
OTA and gut health (absorption, barrier integrity, and immunity).
| Gut function | Subjects | Dose | Time | Results | Reference |
|---|---|---|---|---|---|
| Absorption | HT-29-D4 cells | 100 μmol | 48 h | Na+-dependent glucose absorption was inhibited by 60 ± 4% | Maresca et al., (2001) |
| Cortical astrocytes | 10 μmol | 72 h | OTA could inhibit the absorption of glutamate by decreasing the expression of GLAST and GLT-1 at the cell surface | Razafimanjato et al., (2010) | |
| 1-day-old ducklings | 2 mg/kg diet | 21 d | OTA downregulated the expression of SGLT1, CAT1, EAAT, and PepT1 mRNA in jejunum, and decreased the expression | Ruan (2018) | |
| Barrier integrity | 1-day-old ducklings | 2 mg/kg diet | 21 d | OTA decrease the expression of occludin and tight junction protein 1 at the level of transcription and protein in jejunum | Ruan et al., (2019) |
| 7-day-old ducklings | 235 μg/kg body weight | 14 d | OTA decreased the expression of occludin and tight junction protein 1 at the level of transcription and protein in cecum, and increased the serum LPS content | Wang et al., (2019) | |
| 1-day-old white feather broilers | 50 μg/kg body weight | 21 d | OTA decreased expressions of claudin-1, occludin and ZO-1 at the mRNA level, and decreased the expression of claudin-1 at the protein level | Yang et al., (2020) | |
| Immunity | Male broiler chickens (Ross 308) | 20 and 50 μg/kg body weight | 28 d | OTA reduced the lymphocyte population in the intestinal epithelium and the lamina propria, and increased the CD4+ and CD8+ values in both the duodenum and jejunum after 28 d of administration | Solcan et al., (2015) |
| Ducklings | 2 mg/kg diet | 21 d | OTA induced the secretion of IL-1β and TNF-α in the jejunum and decreased the IgA content in the jejunum | Ruan (2018) | |
| 1-day-old broiler chickens | 50 μg of OTA/kg body weight/d | 21 d | OTA could upregulate the mRNA expression of IL-1β, TNF-α and induce phosphorylation of NF-κB | Tong et al., (2020) |
Abbreviations: CAT1, cationic amino acid transporter 1; EAAT, excitatory amino acid transporter; GLAST, glutamate/aspartate transporter; GLT-1, glutamate transporter 1; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; OTA, ochratoxin A; PepT1, peptide transporter 1; SGLT1, sodium-dependent glutamate transporter 1; TNF, tumor necrosis factor; ZO-1, zonula occludens 1.
Gut Microbiota
The gut microbiota represents an ensemble of microorganisms including bacteria, viruses, and fungi, that are harbored within the GIT of living organisms. The gut microbiota is found in virtually every metazoan, from invertebrates to vertebrates, and it affects numerous physiological functions of the intestine (Lee and Hase, 2014; Subramanian et al., 2014; Ren et al., 2016; Thaiss et al., 2016). The gut microbiota is linked to the pathogenesis of various diseases through the activity of the microbiome or its metabolic products (Louis et al., 2014; Qin et al., 2014; Anhê et al., 2015; Ponziani et al., 2019).
Composition and Functions of the Gut Microbiota
The composition of the gut microbiota varies significantly with the relative ratios of dominant phyla, genera, and species. In particular, stable and healthy gut microbiota is generally indicated by a rich diversity of gut bacteria (Mosca et al., 2016). The majority of the gut microbiota is composed of strict anaerobes, which dominate the facultative anaerobes and aerobes by 2 to 3 orders of magnitude (Gordon and Dubos, 1970; Savage, 1970; Harris et al., 1976). Adult poultry intestinal microbiota are dominated by 2 phyla, Bacteroidetes and Firmicutes, whereas the Actinobacteria (including Bifidobacterium spp.), Proteobacteria (including Escherichia coli and Enterobacteriaceae family members), and Verrucomicrobia (including Akkermansia muciniphila) phyla are present only in minor proportions (Wang et al., 2017; Zhai et al., 2020). The concentration and microbial diversity of the gut microbiota increase from the duodenum to distal colon segments. At the population level, the microbiota is generally stable and selected by evolution (Ochman et al., 2010; Jalanka-Tuovinen et al., 2011). However, commensal microbiota can be qualitatively and quantitatively modulated by the environment (Huttenhower et al., 2012). Diet plays an important role in shaping the gut microbiota (Salonen et al., 2014; Frese et al., 2015). Moreover, genetic background, diseases such as inflammatory bowel disease (Zhang et al., 2017), nonalcoholic fatty liver (Roy et al., 2012), obesity (Kallus and Brandt, 2012), and type 1 diabetes (Vaarala et al., 2008), genetic background (Goodrich et al., 2016), the mental health condition (stress) of the host (Karl et al., 2017), sports (García et al., 2014), and mycotoxin (Piotrowska et al., 2014; Wang et al., 2015) can also alter the composition and proportion of the gut microbiota.
Impact of OTA on the Gut Microbiota
The gut microbiota represents an important bridge between environmental substances and host metabolism. Findings showed that the gut microbiota, particularly in animals, has profound interactions upon the ingestion of mycotoxins. Indeed, the absorption rate of OTA varies from animals to humans (e.g., 66% in pigs, 56% in rabbits, and 40% in chickens [Galtier et al., 1981]). This indicates that most OTA are not absorbed, and thus reach the hindgut and interact with the intestinal microbiota, especially in poultry, which presents a low absorption rate. However, few studies have focused on the interaction between OTA and the gut microbiota. Evidence of disturbance to gut microbiota modulation induced by OTA has been summarized inTable 3.
Table 3.
Ochratoxin A and gut microbiota.
| Subjects | Dose | Time | Results | Reference |
|---|---|---|---|---|
| 1-day-old broilers | 50 μg/kg body wight | 21 d | OTA decreased the richness and diversity of cecum microbiota | Yang et al., (2020) |
| 7-day-old ducklings | 235 μg/kg body wight | 14 d | OTA decreased the richness and diversity of cecum microbiota and increased the relative abundance of cecum Bacteroides | Wang et al., (2019) |
| 1-day-old ducklings | 2 mg/kg diet | 21 d | OTA increased the relative abundance of cecum Bacteroides and decreased the butyric acid producing bacteria | Zhai et al., (2020) |
Abbreviation: OTA, ochratoxin A.
The fate of OTA during colon digestion was investigated using a dynamic simulator of the human gut (Ouethrani et al., 2013). In previous research, gut microbiota degradation of OTA and microbiota diversity alteration were observed only in the descending colon after 1 wk of exposure to OTA, and OTA caused a specific, but lasting, loss of the beneficial species Lactobacillus reuteri (Ouethrani et al., 2013). Studies in broilers and ducks suggested that OTA decreased the richness and diversity of the cecum microbiota and induced intestinal TJ injury (Wang et al., 2019; Yang et al., 2020). Our latest research confirmed these results, which increased the relative abundance of cecum Bacteroides, in ducks fed OTA-contaminated diets (Zhai et al., 2020). Although Bacteroides (gram-negative bacteria) is often associated with leanness and other desirable health traits (Turnbaugh et al., 2006; Ridaura et al., 2013), some of its strains (e.g., Bacteroides fragilis, Bacteroides vulgatus, and Bacteroides dorei) have been linked to abdominal infections, metabolic disease, and inflammation (De Palma et al., 2010; Davis-Richardson et al., 2014). This outcome may further suggest that OTA may cause negative effects on host health via gut microbiota modulation. Moreover, the elevated bacterial strains may have some tolerance to OTA and may be able to degrade or absorb OTA. However, few studies have reported effects of OTA on gut microbiota and microbiota metabolites, especially with chronic exposure at safe doses. Therefore, this area of research needs to be addressed further. Moreover, evaluating the variations in microbial functions such as signal transduction and metabolism by transcriptomics and/or metabolomics might provide great opportunities to assess the impact of OTA on gut health and the development of bacteria and metabolites regulating OTA-induced intestinal injury.
Conclusion
All of the issues raised herein indicate the necessity for determining the deleterious effects of ingested OTA on the epithelium, mucosa, and microbiota of poultry. The gut microbiota can vary within the same species; thus, different results regarding OTA can be observed, as discussed in this article. However, a common consequence is that OTA disrupts the TJs and the gut microbiota balance and thereby dysregulates intestinal functions and impairs local immune response, which may eventually result in systemic toxicity that leads to chronic mycotoxicosis, such as liver inflammation. It will be interesting to study if the pathogenesis of OTA-induced disease in other organs (e.g., brain and kidney injury) depends on the intestinal microbiota.
Although there are many studies on the effects of OTA on the gut health of poultry, the types of gut microbes or metabolites affected by OTA and thus the harm they cause to gut health are not known. Whether there is a maternal effect of OTA on gut health has not been studied. In addition, the effects of chronic exposure to a safe OTA dose in poultry should not be underestimated. This review highlights the important role of the gut microbiota in OTA-induced gut injury in poultry, which may open a new path to novel preventative or therapeutic interventions for mycotoxicosis, such as regulating the intestinal microbiota and improving the intestinal barrier with probiotics.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (32002219), Yangtze University Scientific Research Starting Foundation for 2019 “Double First-Class” (802100270303), Natural Science Foundation of Guangdong Province (2019B1515210012), Guangdong province-Wens Joint Natural Science Foundation (2020117895), and China Agriculture Research System (CARS-42-15).
Disclosures
The authors declare no conflict of interest.
References
- Adetunji M.C., Atanda O.O., Ezekiel C.N. Risk Assessment of mycotoxins in stored maize grains consumed by infants and young children in Nigeria. Children. 2017;4:58. doi: 10.3390/children4070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleo M.D., Wyatt R.D., Schnellmann R.G. Mitochondrial dysfunction is an early event in ochratoxin a but not oosporein toxicity to rat renal proximal tubules. Toxicol. Appl. Pharmacol. 1991;107:73–80. doi: 10.1016/0041-008x(91)90332-9. [DOI] [PubMed] [Google Scholar]
- André E.K., Ali A. Ochratoxin A: General overview and actual molecular status. Toxins. 2010;2:461–493. doi: 10.3390/toxins2040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhê F.F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T.V., Garofalo C., Moine Q., Desjardins Y., Levy E., Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- Assunção R., Martins C., Dupont D., Alvito P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: a contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016;96:205–214. doi: 10.1016/j.fct.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Bhatti S.A., Khan M.Z., Saleemi M.K., Hassan Z. Impact of dietary Trichosporon mycotoxinivorans on ochratoxin A induced immunotoxicity; in vivo study. Food Chem. Toxicol. 2019;132:110696. doi: 10.1016/j.fct.2019.110696. [DOI] [PubMed] [Google Scholar]
- Blanc M., Pittet A., Muñoz-Box R., Viani R. Behavior of ochratoxin A during green coffee roasting and soluble coffee manufacture. J. Agric. Food Chem. 1998;46:673–675. doi: 10.1021/jf9707703. [DOI] [PubMed] [Google Scholar]
- Boudra H., Bars P.L., Bars J.L. Thermostability of Ochratoxin A in wheat under two moisture conditions. Appl. Environ. Microb. 1995;61:1156–1158. doi: 10.1128/aem.61.3.1156-1158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C.T., Farkas A.E., Nusrat A. Epithelial adhesive junctions. F1000 Prime Rep. 2014;6:1. doi: 10.12703/P6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G.A., Arntzen C.J. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Am. J. Physiol. 1993;265:G599–G610. doi: 10.1152/ajpgi.1993.265.4.G599. [DOI] [PubMed] [Google Scholar]
- Chen A., Jiao X., Hu Y., Lu X., Gao W. Mycobiota and mycotoxins in traditional medicinal seeds from China. Toxins. 2015;7:3858–3875. doi: 10.3390/toxins7103858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F.S. Interaction of ochratoxin A with bovine serum albumin. Arch. Biochem. Biophys. 1971;147:359–366. doi: 10.1016/0003-9861(71)90391-2. [DOI] [PubMed] [Google Scholar]
- Chu F.S. A comparative study of the interaction of ochratoxins with bovine serum albumin. Biochem. Pharmacol. 1974;23:1105–1113. doi: 10.1016/0006-2952(74)90011-2. [DOI] [PubMed] [Google Scholar]
- Constantinescu C.S., Chou I.J. Springer, Cham, Switzerland; 2016. Intestinal Bacterial Antigens, Toxin-Induced Pathogenesis and Immune Cross-Reactivity in Neuromyelitis Optica and Multiple Sclerosis. [Google Scholar]
- Davis-Richardson A.G., Ardissone A.N., Dias R., Simell V., Leonard M.T., Kemppainen K.M., Drew J.C., Schatz D., Atkinson M., Kolaczkowski B., Ilonen J., Knip M., Toppari J., Nurminen N., Hyöty H., Beijola R., Simell T., Mykkänen J., Simell O., Triplett E. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front. Microbiol. 2014;5:678. doi: 10.3389/fmicb.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G., Nadal I., Medina M., Donat E., Ribes-Koninckx C., Calabuig M., Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. Bmc Microbiol. 2010;10:63. doi: 10.1186/1471-2180-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union Commission Recommendation No 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, Ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding, (Text with EEA relevance) Off. J. Eur. Union, C. 2006;229:7–9. [Google Scholar]
- Fink-Gremmels J. Conclusions from the workshops on ochratoxin A in food: Recent developments and significance, organized by ILSI Europe in Baden (Austria), 29 June-1 july 2005. Food Addit. Contam. 2005;22:1–5. [Google Scholar]
- Fink J., Koo B.K. Vol. 908. Springer; Cham, Switzerland: 2016. pp. 11–25. (in Clonal evolution of stem cells in the gastrointestinal tract. Stem Cells, Pre-neoplasia, and Early Cancer of the Upper Gastrointestinal Tract). [DOI] [PubMed] [Google Scholar]
- Frantisek M., Vladimir O., Annie P.L., Jan M., Jakub T. Ochratoxin A: 50 years of research. Toxins. 2016;8:191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese S.A., Parker K., Calvert C.C., Mills D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano N., Donne I.D., Torri C., Migliori M., Gioia M. Early cytotoxic effects of ochratoxin A in rat liver: a morphological, biochemical and molecular study. Toxicology. 2006;225:214–224. doi: 10.1016/j.tox.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Galtier P., Alvinerie M., Charpenteau J.L. The pharmacokinetic profiles of ochratoxin A in pigs, rabbits and chickens. Food Cosmet. Toxicol. 1981;19:735–738. doi: 10.1016/0015-6264(81)90528-9. [DOI] [PubMed] [Google Scholar]
- Gambacorta L., Magistà D., Perrone G., Murgolo S., Solfrizzo M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018;11:1–16. [Google Scholar]
- García J.A.V. Intestinal microbiota and intense physical activity. Archivos De Medicina Del. Deporte. 2014;31:268–272. [Google Scholar]
- Gerrit H.D., van der Stegen, Paulus J.M.E., Joost V.D.L. Effect of roasting conditions on reduction of ochratoxin A in coffee. J. Agric. Food Chem. 2001;49:4713–4715. doi: 10.1021/jf0105586. [DOI] [PubMed] [Google Scholar]
- Gordon J.H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J. Exp. Med. 1970;132:251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J.K., Davenport E.R., Waters J.L., Clark A.G., Ley R.E. Cross-species comparisons of host genetic associations with the microbiome. Science. 2016;352:532–535. doi: 10.1126/science.aad9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B., Applegate T. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B., Oswald I. Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4:285–313. [Google Scholar]
- Gumus R., Ercan N., Imik H. Determination of Ochratoxin A levels in mixed feed and feed stuffs used in some laying hens and ruminant enterprises of Sivas city. Revista Brasileira de Ciência Avícola. 2018;20:85–90. [Google Scholar]
- Hagelberg S., Hult K., Fuchs R. Toxicokinetics of ochratoxin A in several species and its plasma-binding properties. J. Appl. Toxicol. 2010;9:91–96. doi: 10.1002/jat.2550090204. [DOI] [PubMed] [Google Scholar]
- Hameed M.R.M., Khan M., Kashif A., Khan M., Akhtar Z., Hassan Z.H. Study of ochratoxin A (OTA)-induced oxidative stress markers in broiler chicks. Toxin Rev. 2017;36:1–5. [Google Scholar]
- Harris M.A., Reddy C.A., Carter G.R. Anaerobic bacteria from the large intestine of mice. Appl. Environ. Microb. 1976;31:907–912. doi: 10.1128/aem.31.6.907-912.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Z., Khan M.Z., Khan A., Javed I., Hussain Z. Effects of individual and combined administration of ochratoxin A and aflatoxin B 1 in tissues and eggs of White Leghorn breeder hens. J. Sci. Food Agri. 2012;92:1540–1544. doi: 10.1002/jsfa.4740. [DOI] [PubMed] [Google Scholar]
- Hassan Z., Khan M.Z., Saleemi M.K., Khan A., Javed I., Bhatti S.A. Toxico-pathological effects of in ovo inoculation of ochratoxin A (OTA) in chick embryos and subsequently in hatched chicks. Toxicolo. Pathol. 2012;40:33–39. doi: 10.1177/0192623311425058. [DOI] [PubMed] [Google Scholar]
- Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J.H. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S.Z., Nisar S., Asi M.R., Jinap S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control. 2014;43:98–103. [Google Scholar]
- Jahanian E. Mycotoxin-induced toxicity: an updated mini-review on the current concepts. Immuno. Persa. 2016;2:e11. [Google Scholar]
- Jaladanki R.N., Wang J. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2nd ed. Morgan & Claypool Publishers; San Rafael, CA: 2016. Regulation of gastrointestinal mucosal growth; pp. 1–132. [Google Scholar]
- Jalanka-Tuovinen J., Salonen A., Nikkila J., Immonen O., Kekkonen R., Lahti L., Palva A., de Vos W.M. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011;6:e23035. doi: 10.1371/journal.pone.0023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallus S.J., Brandt L.J. The intestinal microbiota and obesity. J. Clin. Gastroenterol. 2012;46:16–24. doi: 10.1097/MCG.0b013e31823711fd. [DOI] [PubMed] [Google Scholar]
- Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y., Hoke A.V., Levangie M.W., Kumar R., Chakraborty N., Gautam A., Hammamieh R., Martini S., Pasiakos S.M. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiologic stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neur. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli P., Najafi M.L., Bahaabadi G.A., Shakeri F., Naghibzadeh A. Evaluation of aflatoxin contamination in raw and roasted nuts in consumed Kerman and effect of roasting, packaging and storage conditions. Life Sci. J. 2014;10(8s):578–583. [Google Scholar]
- Kumagai S. Ochratoxin A: plasma concentration and excretion into bile and urine in albumin-deficient rats. Food Chem. Toxicol. 1985;23:941–943. doi: 10.1016/0278-6915(85)90112-7. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Li Y.L. Sichuan Agricultural University; Yaan, China: 2016. Mycotoxin Distribution of Poultry Compound Feed in Southwest China. [Google Scholar]
- Liuzzi V.C., Fanelli F., Tristezza M., Haidukowski M., Picardi E., Manzari C., Lionetti C., Grieco F., Logrieco A.F., Thon M.R., Pesole G., Mulè G. Transcriptional analysis of acinetobacter sp. neg1 capable of degrading ochratoxin A. Front. Microbiol. 2016;7:2162–2170. doi: 10.3389/fmicb.2016.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Magnoli C.E., Astoreca A., Chiacchiera S.M., Dalcero A.M. Occurrence of ochratoxin A and ochratoxigenic mycoflora in corn and corn based foods and feeds in some South American countries. Mycopathologia. 2007;163:249–260. doi: 10.1007/s11046-007-9005-z. [DOI] [PubMed] [Google Scholar]
- Malekinezhad P., Ellestad L.E., Afzali N., Farhangfar S.H., Omidi A., Mohammadi A. Evaluation of berberine efficacy in reducing the effects of aflatoxin B1 and ochratoxin A added to male broiler rations. Poul. Sci. 2020 doi: 10.1016/j.psj.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manizan A., Oplatowska-Stachowiak M., Piro-Metayer I., Campbell K., Koffi-Nevry R., Elliott C., Akaki D., Braber C. Multi-mycotoxin determination in rice, maize and peanut products most consumed in Cote d'Ivoire by UHPLC-MS/MS. Food Control. 2018;87:22–30. [Google Scholar]
- Maresca M., Mahfoud R., Pfohl-Leszkowicz A., Fantini J. The mycotoxin ochratoxin A alters intestinal barrier and absorption functions but has no effect on chloride secretion. Toxicol. Appl. Pharmacol. 2001;176:54–63. doi: 10.1006/taap.2001.9254. [DOI] [PubMed] [Google Scholar]
- Marie-Caroline S., Stéphanie M., Emmanuel C., Nolwenn H. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins. 2016;8:94. doi: 10.3390/toxins8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mario V., Katherine M., Carolina S., Mario A., Victor C., Ricardo V., Orialis V. Solid-phase extraction and HPLC determination of Ochratoxin A in cereals products on Chilean market. Food Control. 2009;20:631–634. [Google Scholar]
- Mata A.T., Ferreira J.P., Oliveira B.R., Batoreu M.C., Crespo M.T.B., Pereira V.J., Bronze M.R. Bottled water: analysis of mycotoxins by LC-MS/MS. Food Chem. 2015;176:455–464. doi: 10.1016/j.foodchem.2014.12.088. [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Hasnain S.Z. Goblet cells as mucosal sentinels for immunity. Mucosal Immunol. 2017;10:1118–1121. doi: 10.1038/mi.2016.132. [DOI] [PubMed] [Google Scholar]
- Medina Á., Rodríguez A., Magan N. Climate change and mycotoxigenic fungi: impacts on mycotoxin production. Curr. Opin. Food Sci. 2015;5:99–104. [Google Scholar]
- Mohd-Redzwan S., Jamaluddin R.A., Mutalib M.S., Ahmad Z. A mini review on aflatoxin exposure in Malaysia: past, present and future. Front. Microbiol. 2013;4:334. doi: 10.3389/fmicb.2013.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca A., Leclerc M., Hugo J.P. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front. Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Worobey M., Kuo C.H., Ndjango J.B., Peeters M., Hahn B.H., Hugenholtz P. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 2010;8:e1000546. doi: 10.1371/journal.pbio.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouethrani M., Wiele T.V.D., Verbeke E., Bruneau A., Carvalho M., Rabot S., Camel V. Metabolic fate of ochratoxin A as a coffee contaminant in a dynamic simulator of the human colon. Food Chem. 2013;141:3291–3300. doi: 10.1016/j.foodchem.2013.05.157. [DOI] [PubMed] [Google Scholar]
- Pandya J.P., Arade P.C. Mycotoxin: a devil of human, animal and crop health. Adv. Life Sci. 2016;5:3937–3941. [Google Scholar]
- Parvin D., Keyvan P., Hossein R., Mostafa C., Mojtaba A. Prevalence of ochratoxin A in human milk in the Khorrambid Town, Fars province, South of Iran. Jundishapur J. Microb. 2014;7:e11220. doi: 10.5812/jjm.11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham J.C., Doupnik B.J., Jones O.H. Acute toxicity of ochratoxins A and B in chicks. Appl. Microbiol. 1971;21:492–494. doi: 10.1128/am.21.3.492-494.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietro C.L., Aaron C., Fru-Nji F., Robert E.S., Anna-Maria K., Viviane V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Tech. 2017;234:88–100. [Google Scholar]
- Piotrowska M., Śliżewska K., Nowak A., Zielonka Ł., Żakowska Z., Gajęcka M., Gajęcki M. The effect of experimental fusarium mycotoxicosis on microbiota diversity in porcine ascending colon contents. Toxins. 2014;6:2064–2081. doi: 10.3390/toxins6072064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plestina R. Enterohepatic circulation of ochratoxin A in rats. Period. Biol. 1988;90:39–42. [Google Scholar]
- Pohland A.E., Schuller P.L., Steyn P.S., Egmond H.P.V. Physicochemical data for some selected mycotoxins. Pure Appl. Chem. 1982;54:2219–2284. [Google Scholar]
- Ponziani F.R., Bhoori S., Castelli C., Putignani L., Rivoltini L., Del Chierico F., Sanguinetti M., Morelli D., Sterbini F.P., Petito V., Reddel S., Calvani R., Camisaschi C., Picca A., Tuccitto A., Gasbarrini A., Pompili M., Mazzaferro V. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- Prior M.G., Sisodia C.S., O’Neil J.B. Acute oral ochratoxicosis in day-old White Leghorns, turkeys and Japanese quail. Poul. Sci. 1976;55:786–790. doi: 10.3382/ps.0550786. [DOI] [PubMed] [Google Scholar]
- Purchase I.F.H., Theron J.J. The acute toxicity of ochratoxin A to rats. Food Cosmet. Toxicol. 1968;6:479–483. doi: 10.1016/0015-6264(68)90138-7. [DOI] [PubMed] [Google Scholar]
- Qin N., Yang F., Li A., Prifti E., Chen Y., Shao L., Guo J., Le Chatelier E., Yao J., Wu L., Zhou J., Ni S., Liu L., Pons N., Batto J.M., Kennedy S.P., Leonard P., Yuan C., Ding W., Chen Y., Hu X., Zheng B., Qian G., Xu W., Ehrlich S.D., Zheng S., Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- Razafimanjato H., Garmy N., Guo X.J., Varini K., Scala C.D., Pasquale E.D., Taïeb N., Maresca M. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. Neurotoxicology. 2010;31:475–484. doi: 10.1016/j.neuro.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ren W.K., Wang K., Yin J., Chen S., Liu G., Tan B., Wu G.Y., Bazer F.W., Peng Y.Y., Yin Y.L. Glutamine-induced secretion of intestinal secretory immunoglobulin A: a mechanistic perspective. Front. Immunol. 2016;7:503. doi: 10.3389/fimmu.2016.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L., Griffin N.W., Lombard V., Henrissat B., Bain J.R., Muehlbauer M.J., Ilkayeva O., Semenkovich C.F., Funai K., Hayashi D., Lyle B.J., Martini M.C., Ursell L.K., Clemente J.C., Van Treuren W., Walters W.A., Knight R., Newgard C.B., Heath A.C., Gordon J.I. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1049–1079. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringot D., Chango A., Schneider Y.J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006;5:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- Roth A., Chakor K., Ekuécreepy E., Kane A., Roschenthaler R., Dirheimer G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology. 1988;48:293–308. doi: 10.1016/0300-483x(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Roy T.L., Llopis M., Lepage P., Bruneau A., Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2012;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- Ruan D. South China Agricultural University; Guangzhou, China: 2018. Study on the Related Mechanism of Curcumin Ameliorating Ochratoxin A–Induced Intestinal Barrier Injury in Ducks. [Google Scholar]
- Ruan D., Wang W.C., Lin C.X., Fouad A.M., Chen W., Xia W.G., Wang S.Q., Luo X., Zhang W.H., Yan S.J., Zheng C.T., Yang L. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. 2019;13:42–52. doi: 10.1017/S1751731118000678. [DOI] [PubMed] [Google Scholar]
- Salonen A., Lahti L.M., Salojärvi J., Holtrop G., Korpela K., Duncan S.H., Date P., Farquharson F., Johnstone A.M., Lobley G.E., Louis P., Flint H.J., de Vos W.M. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. Isme J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D.C. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am. J. Clin. Nutr. 1970;23:1495–1501. doi: 10.1093/ajcn/23.11.1495. [DOI] [PubMed] [Google Scholar]
- Sherif O., Salama E.E., Abodel-Wahhab M.A. Mycotoxins and child health: the need for health risk assessment. Int. J. Hygenviron Health. 2009;212:347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Shim W.B., Ha K.S., Kim M.G., Kim J.S., Chung D. Evaluation of the transfer rate of ochratoxin a to decoctions of herbal medicines. Food Sci. Biotechnol. 2014;23:2103–2108. [Google Scholar]
- Solcan C., Pavel G., Floristean V., Chiriac I., Lencu B., Solcan G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta Vet. Hung. 2015;63:30–48. doi: 10.1556/AVet.2015.004. [DOI] [PubMed] [Google Scholar]
- Solfrizzo M., Piemontese L., Gambacorta L., Zivoli R., Longobardi F. Food coloring agents and plant food supplements derived from Vitis vinifera: a new source of human exposure to ochratoxin A. J. Agric. Food Chem. 2015;63:3609–3614. doi: 10.1021/acs.jafc.5b00326. [DOI] [PubMed] [Google Scholar]
- Sreemannarayana O., Frohlich A.A., Vitti T.G., Marquardt R.R., Abramson D. Studies of the tolerance and disposition of ochratoxin A in young calves. J. Anim. Sci. 1988;66:1703–1711. doi: 10.2527/jas1988.6671703x. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Huq S., Yatsunenko T., Haque R., Mahfuz M., Alam M.A., Benezra A., DeStefano J., Meier M.F., Muegge B.D., Barratt M.J., VanArendonk L.G., Zhang Q., Province M.A., Petri W.A.J., Ahmed T., Gordon J.I. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Tong C., Li P., Yu L., Li L., Long M. Selenium-rich yeast attenuates ochratoxin A-induced small intestinal injury in broiler chickens by activating the Nrf2 pathway and inhibiting NF-κB activation. J. Funct. Foods. 2020;66:103784. [Google Scholar]
- Trivedi A.B., Doi E., Kitabatake N. Detoxification of ochratoxin A on heating under acidic and alkaline conditions. Biosci. Biotechnol. Biochem. 1992;56:741–745. doi: 10.1271/bbb.56.741. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., J. I. Gordon An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vaarala O., Atkinson M.A., Neu J. The "Perfect Storm" for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Merwe K.J., Steyn P.S., Fourie L., Scott D.B., Theron J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- Veprikova Z., Zachariasova M., Dzuman Z., Zachariasova A., Fenclova M., Slavikova P., Vaclavikova M., Mastovska K., Hengst D., Hajsolva J. Mycotoxins in plant-based dietary supplements: Hidden health risk for consumers. J. Agric. Food Chem. 2015;63:6633–6643. doi: 10.1021/acs.jafc.5b02105. [DOI] [PubMed] [Google Scholar]
- Verma J., Johri T.S., Swain B.K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilisation in white leghorn laying hens. J. Sci. Food Agric. 2007;87:760–764. [Google Scholar]
- Vidal A., Sanchis V., Ramos A.J., Marín S. Thermal stability and kinetics of degradation of deoxynivalenol, deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chem. 2015;178:276–286. doi: 10.1016/j.foodchem.2015.01.098. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang L., Glenn T.C., Wang J.S. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicol. Sci. 2015;150:54–63. doi: 10.1093/toxsci/kfv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhai S., Xia Y., Wang H., Ruan D., Zhou T., Zhu Y., Zhang H., Zhang M., Ye H., Ren W., Yang L. Ochratoxin A induces liver inflammation: involvement of intestinal microbiota. Microbiome. 2019;7:151. doi: 10.1186/s40168-019-0761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zheng S., Sharshov K., Sun H., Yang F., Wang X., Li L., Xiao Z. Metagenomic profiling of gut microbial communities in both wild and artificially reared Bar-headed goose (Anser indicus) Microbiologyopen. 2017;6:e429. doi: 10.1002/mbo3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C.P., Gong Y.Y. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Li L., Yu L., Sun L., Li K., Tong C., Xu W.X., Cui G.Y., Long M., Li P. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem. Toxicol. 2020;137:111139. doi: 10.1016/j.fct.2020.111139. [DOI] [PubMed] [Google Scholar]
- Zhai S., Ruan D., Zhu Y., Li M., Ye H., Wang W., Yang L. Protective effect of curcumin on ochratoxin A-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020;99:1124–1134. doi: 10.1016/j.psj.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cao X., He H. Sampling strategies for three-dimensional spatial community structures in IBD microbiota research. Front. Cell Infect. Microbiol. 2017;7:51. doi: 10.3389/fcimb.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.C. Chinese Academy of Agricultural Sciences; Beijing, China: 2018. Combined Intestinal Toxicity Mechanisms of AFB1 and AFM1 in Mice. [Google Scholar]