Abstract
Background and purpose
Previous studies have reported conflicting results as to whether women have poorer functional outcome than men after thrombolytic therapy. This study aims to investigate the relationship between sex differences and the prognosis of intravenous thrombolysis in Chinese patients with acute ischaemic stroke.
Methods
The patients enrolled in this study were from the Chinese Acute Ischemic Stroke Thrombolysis Monitoring and Registration study. The primary outcome was poor functional outcome, defined as a 3-month modified Rankin score of 3–6. The safe outcome was symptomatic intracranial haemorrhage (SICH) and mortality within 7 days and 90 days. Multiple Cox regression model was used to correct the potential covariates to evaluate the association between sex disparities and prognosis. Furthermore, the interaction of preonset Rankin scores, baseline National Institute of Health Stroke Scale (NIHSS) scores and Trial of Org 10172 in Acute Stroke Treatment (TOAST) types was statistically analysed.
Results
A total of 1440 patients were recruited, including 541 women and 899 men. The baseline information indicated that women were older at the time of onset (66.2±11.2 years vs 61.0±11.3 years, p<0.001), and more likely to have a history of atrial fibrillation (25.3% vs 11.2%, p<0.001), and had a higher NIHSS score on admission (12.3±6.8 vs 11.6±6.7, p=0.04). According to the prognosis analysis of unsatisfactory functional recovery, there was no significant difference between women and men (45.9% vs 37.1%; adjusted OR 1.01, 95% CI 0.75 to 1.37). As for the safe outcome, the proportion of SICH and mortality in women is relatively high but did not reach statistical significance. There was no significant interaction with sex, age, preonset Rankin score, NIHSS score, TOAST classification and the prognosis of intravenous thrombolysis.
Conclusions
For Chinese patients with ischaemic stroke, although women are older and more severe at the time of onset, the prognosis after intravenous thrombolysis is not significantly different from men.
Keywords: stroke, thrombolysis
Introduction
Sex differences in biological and gender differences in sociology and culture affect epidemiology, pathophysiology, treatment and prognosis of cardiovascular diseases.1 It is well known that distinctive expression of sex chromosomes and peculiar sexual hormones leads to different physiology and pathophysiology processes among men and women, such as immunity and coagulation.2 3 And compared with men, women have some unique or more prevalent stroke risk factors, such as pre-eclampsia, atrial fibrillation and migraine.4–6 However, multiple evidence from previous studies showed that women have a higher survival rate, but weaker functional recovery, higher disability rate and poorer quality of life compared with men.7 8 Therefore, the American Heart Association/American Stroke Association have issued guidelines on stroke prevention for women.9
The proportion of women among the prevalent cases of stroke globally is higher than men in general.10 11 Confronting aggravating process of ageing, analysing the influences of sex differences on prevention and treatment of stroke will have a remarkable impact on reducing global health burden.12
Intravenous thrombolysis is one of the most effective treatments for patients with acute ischaemic stroke (AIS), accompanying with increased risk of bleeding events disappointedly.13 Recent work showed that women are more responsive to intravenous tissue plasminogen activator (tPA).14 However, studies are still controversial about sex-related functional outcomes, symptomatic intracranial haemorrhage (SICH) rate and mortality of patients with AIS after thrombolytic therapy.15–17 Since most acute stroke studies were not mainly designed to assess sex effection, it is tough to adjust the results by sex-specific risk factors, such as premorbid functional status or door-to-needle times. The analysis of the correlation between sex and prognosis after thrombolysis is partly not comprehensive. Thus, we collected and analysed the data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China) to figure out new evidence on sex differences in prognosis after intravenous thrombolysis in Chinese patients.
Methods
Study design
TIMS-China was a nationwide prospective stroke registry programme enrolling patients with AIS (18–80 years old; platelet ≥100 000/mm3) who received intravenous tPA within 4.5 hours after symptom onset in China.18 Recruitment of patients in TIMS-China opened in May 2007, and closed in April 2012. Sixty-seven major stroke centres participated. Details of the trial design and some results have been reported in the previous literature.19 20 The ethics committee approved the study protocol of Beijing Tiantan Hospital with the Helsinki Declaration. The registry was regularly monitored by the quality monitoring committee of TIMS-China and the Contract Research Organization independently. Each participant signed informed consent, and was followed up for 3 months. According to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, all patients were divided into five subtypes: large-artery atherosclerosis (LAA), cardioembolism (CE), small-artery occlusion, stroke of other determined cause and stroke of undetermined cause.21
Clinical outcome measurement
The primary outcome was functional recovery assessed with the modified Rankin Scale (mRS) at 3 months. The poor functional outcome was defined as disability/death (mRS score of 3–6), while the good functional outcome was defined as excellent recovery (mRS score of 0–1) and functional independence (mRS score of 0–2).22
The secondary outcomes included SICH and mortality after thrombolysis within 7 days and 90 days. Depending on previous trials, SICH was evaluated by the following three definitions: (1) safe implementation of treatments in stroke-monitoring study: local or remote parenchymal haemorrhage type 2 on the 22–36 hours post-treatment imaging scan, combined with a neurological deterioration of ≥4 points on the National Institute of Health Stroke Scale (NIHSS) from baseline, or the lowest NIHSS value between baseline and 24 hours, or leading to death22; (2) National Institute of Neurological Disorders and Stroke: a haemorrhage that was not present on previous CT scans, and there had subsequently been either a suspicion of haemorrhage or any decline in neurological status23; (3) second European–Australasian acute stroke study: any intracranial bleeding, with ≥4 points clinical worsening on NIHSS score.24
Statistical analysis
Continuous variables were presented as mean±SD or medians (IQR) and categorical variables as percentages. The baseline characteristics of different sex were compared by Wilcoxon rank-sum tests for continuous variables and χ2 test for categorical variables. The ORs with their 95% CIs and the adjusted ORs with their 95% CI were calculated using univariate and multivariate logistic regression. Adjusted variables included age, diastolic blood pressure (BP), current smoking status, NIHSS on admission and the baseline variables significantly differed (p<0.05) between women and men. The different distribution of mRS was tested by multiple linear regression. Wilcoxon rank-sum test was used to compare the improvement of NIHSS score between different sex, and multiple linear regression was used to correct it. The interaction of preonset Rankin scores, baseline NIHSS scores and TOAST types was analysed by the multivariate Cox model. Statistical significance was determined as p<0.05, two sided. SAS 9.4 (SAS Institute) was used for all statistical analyses.
Results
Baseline characteristics
A total of 1440 consecutive patients who received intravenous thrombolysis were enrolled in this study, among which 541 (37.6%) were women. Table 1 shows the demographics and clinical characteristics of the patients by sex. Compared with men, women were older, more likely to have a history of atrial fibrillation, hypertension and diabetes mellitus, but less likely to be current smokers. Meanwhile, women were more severe on admission. However, no matter the stroke-to-needle time or the stroke-to-visit time, men were longer than women. As for TOAST types, the proportion of CE in women was higher than in men (26.6% vs 13.0%). There was no difference in total stay time.
Table 1.
Demographics and clinical characteristics of patients in Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (N=1440)
| Women (n=541) | Men (n=899) | P value | |
| Age, years, mean (SD) | 66.2±11.2 | 61.0±11.3 | <0.001 |
| Clinical features | |||
| Systolic BP, mm Hg (IQR) | 150(135-162) | 148(132-162) | 0.24 |
| Diastolic BP, mm Hg (IQR) | 85 (78–91) | 89 (80–97) | <0.001 |
| Glucose, mmol/L (IQR) | 6.8 (5.8–8.8) | 6.8 (5.8–8.4) | 0.06 |
| Current smoking, n (%) | 25 (4.6) | 490 (54.4) | <0.001 |
| Medical history | |||
| Previous ischaemic stroke, n (%) | 96 (17.7) | 151 (16.8) | 0.64 |
| Previous TIA, n (%) | 42 (9.3) | 84 (9.3) | 0.30 |
| Hypertension, n (%) | 337 (62.3) | 507 (56.4) | 0.03 |
| Hyperlipidaemia, n (%) | 35 (6.5) | 59 (6.6) | 0.94 |
| Diabetes mellitus, n (%) | 116 (21.4) | 140 (15.6) | 0.005 |
| Atrial fibrillation, n (%) | 137 (25.3) | 101 (11.2) | <0.001 |
| Baseline medication | |||
| Antiplatelets use before stroke, n (%) | 79 (14.6) | 112 (12.5) | 0.25 |
| Anticoagulants use before stroke, n (%) | 9 (1.7) | 15 (1.7) | 0.99 |
| Antihypertensive agents before stroke, n (%) | 236 (43.6) | 296 (32.9) | <0.001 |
| Prestroke mRS 0–2, n (%) | 528 (97.8) | 884 (98.7) | 0.21 |
| Median NIHSS on admission, mean (SD) | 12.3±6.8 | 11.6±6.7 | 0.04 |
| Median stroke-to-visit time, min (IQR) | 1.2 (0.7–2.0) | 1.3 (0.8–2.2) | 0.04 |
| Median stroke-to-imaging time, min (IQR) | 1.9 (1.3–2.9) | 1.8 (1.3–2.6) | 0.03 |
| Median stroke-to-needle time, min | 0.04 | ||
| 0–3 hours, n (%) | 305 (56.5) | 449 (50.2) | |
| 3–4.5 hours, n (%) | 135 (25.0) | 239 (26.7) | |
| >4.5 hours, n (%) | 100 (18.5) | 207 (23.1) | |
| TOAST type | <0.001 | ||
| LAA, n (%) | 272 (50.5) | 549 (61.7) | |
| SAO, n (%) | 44 (8.2) | 83 (9.3) | |
| CE, n (%) | 143 (26.6) | 116 (13.0) | |
| Other, n (%) | 79 (14.7) | 142 (16.0) | |
| Total length of stay (days) | 15 (11–21) | 14 (11–21) | 0.10 |
BP, blood pressure; CE, cardioembolism; LAA, large-artery atherosclerosis; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SAO, small-artery occlusion; TIA, transient ischaemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Clinical outcomes
There were 1410 patients (97.9%) who had a 3-month mRS score. The proportion of poor function outcomes is 45.9% for women and 37.1% for men (crude OR 1.44; 95% CI 1.16 to 1.79). After adjustment for age, diastolic BP, current smoking, hypertension, diabetes mellitus, atrial fibrillation, antihypertensive agents before stroke, stroke-to-needle time, stroke-to-visit time, stroke-to-imaging time, NIHSS on admission and TOAST types, there was no statistical difference (adjusted OR 1.01; 95% CI 0.75 to 1.37; table 2). Further analysis of the relationship between sex, age, preonset mRS score, NIHSS on admission and TOAST types, there was no interaction in poor functional outcome after thrombolysis of patients with AIS (table 3). After age 50 years, the rate of poor functional outcomes in women was somewhat higher than that in men, but no significance was found in further age stratification analysis (table 3; online supplementary tables 1-3).
Table 2.
Outcomes after intravenous thrombolysis in women versus men
| Outcome | No. (%) of patients | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | |
| Women (n=529) | Men (n=881) | |||||
| Primary outcomes | ||||||
| mRS 3–6 at 3 months | 243 (45.9) | 327 (37.1) | 1.44 (1.16 to 1.79) | 0.001 | 1.01 (0.75 to 1.37) | 0.95 |
| Safety outcomes | ||||||
| SICH (SITS-MOST) | 12 (2.4) | 15 (1.7) | 1.45 (0.69 to 3.07) | 0.33 | 1.26 (0.50 to 3.21) | 0.63 |
| SICH (NINDS) | 35 (6.5) | 43 (4.8) | 1.15 (0.66 to 2.00) | 0.62 | 0.89 (0.51 to 1.55) | 0.67 |
| SICH (ECASS II) | 22 (4.1) | 32 (3.6) | 1.38 (0.87 to 2.18) | 0.17 | 0.82 (0.42 to 1.61) | 0.56 |
| Mortality at 7 days | 32 (5.9) | 43 (4.8) | 1.25 (0.78 to 2.00) | 0.35 | 0.94 (0.51 to 1.71) | 0.83 |
| Mortality at 3 months | 60 (11.3) | 81 (9.2) | 1.26 (0.88 to 1.79) | 0.20 | 0.79 (0.50 to 1.26) | 0.32 |
*Adjusted baseline variables: age, diastolic blood pressure, current smoking, hypertension, diabetes mellitus, atrial fibrillation, antihypertensive agents before stroke, stroke-to-needle time, stroke-to-visit time, stroke-to-imaging time, National Institute of Health Stroke Scale (NIHSS) on admission and Trial of Org 10172 in Acute Stroke Treatment (TOAST) types.
ECASS II, second European–Australasian acute stroke study; mRS, modified Rankin Scale; NINDS, National Institute of Neurological Disorders and Stroke; SICH, symptomatic intracranial haemorrhage; SITS-MOST, safe implementation of treatments in stroke-monitoring study.
Table 3.
Interaction with poor functional outcome after intravenous thrombolysis in women versus men
| Women n (%) | Men n (%) | mRS score of 3–6 unadjusted OR (95% CI) |
P value | Interaction p values |
mRS score of 3–6 adjusted OR (95% CI) * |
P value | Interaction p values |
|
| Age<50 years | 17 (34.7) | 31 (22.3) | 1.85 (0.91 to 3.77) | 0.09 | 0.40 | 1.57 (0.52 to 4.76) | 0.42 | 0.53 |
| Age≥50 years | 226 (47.1) | 296 (39.9) | 1.34 (1.06 to 1.69) | 0.01 | 1.00 (0.74 to 1.37) | 0.98 | ||
| Pre-mRS≤2 | 238 (46.1) | 319 (36.8) | 1.47 (1.18 to 1.83) | 0.0007 | 0.21 | 1.02 (0.75 to 1.39) | 0.89 | 0.56 |
| Pre-mRS>2 | 5 (41.7) | 7 (58.3) | 0.51 (0.10 to 2.59) | 0.42 | – | – | ||
| NIHSS≤5 | 11 (13.4) | 14 (9.2) | 1.53 (0.66 to 3.54) | 0.32 | 0.89 | 0.83 (0.26 to 2.68) | 0.75 | 0.97 |
| NIHSS>5 | 232 (51.9) | 313 (42.9) | 1.43 (1.13 to 1.82) | 0.003 | 1.05 (0.78 to 1.41) | 0.76 | ||
| TOAST types | 0.54 | 0.48 | ||||||
| LAA | 123 (46.2) | 214 (40.0) | 1.30 (0.97 to 1.75) | 0.08 | 0.95 (0.63 to 1.42) | 0.8 | ||
| SAO | 10 (22.7) | 15 (18.5) | 1.29 (0.53 to 3.19) | 0.57 | 0.19 (0.04 to 0.95) | 0.04 | ||
| CE | 81 (57.9) | 62 (53.5) | 1.20 (0.73 to 1.96) | 0.48 | 1.14 (0.6 to 2.19) | 0.68 | ||
| Others | 28 (36.8) | 30 (21.9) | 2.08 (1.12 to 3.86) | 0.02 | 1.47 (0.61 to 3.55) | 0.39 |
*Adjusted baseline variables: age, diastolic blood pressure, current smoking, hypertension, diabetes mellitus, atrial fibrillation, antihypertensive agents before stroke, stroke-to-needle time, stroke-to-visit time, stroke-to-imaging time, National Institute of Health Stroke Scale (NIHSS) on admission and Trial of Org 10172 in Acute Stroke Treatment (TOAST) types, except the analysis factor itself.
CE, cardioembolism; LAA, large-artery atherosclerosis; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SAO, small-artery occlusion; TOAST, the Trial of Org 10172 in Acute Stroke Treatment.
svn-2020-000351supp001.pdf (90.5KB, pdf)
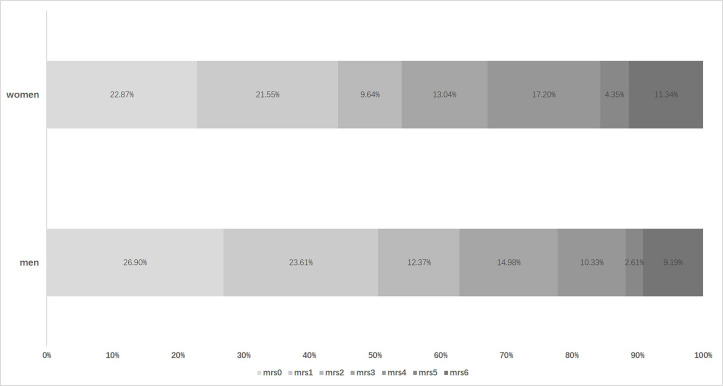
Although there was a numerical difference in the distribution of 3-month mRS among patients of different sex, it did not reach a statistical difference after adjustment (crude p=0.001, adjusted p=0.78; figure 1). Table 4 shows the NIHSS scores in different periods after thrombolysis. The NIHSS score of women was higher on admission and 2 hours after thrombolysis (on admission, 12.3±6.8 vs 11.6±6.7, p=0.04; 2 hours, 9.8±7.0 vs 9.0±7.2, p=0.004). But there was no difference in NIHSS scores between women and men in 24 hours and 7 days after thrombolysis (24 hours, 9.1±8.3 vs 8.3±7.9, p=0.07; 7 days, 6.7±7.8 vs 6.2±7.0, p=0.41). Moreover, NIHSS improving scores, that is, using of NIHSS score on admission to minus NIHSS score at different times, were similar between men and women.
Figure 1.
Distribution of modified Rankin scale (mRS) score after intravenous thrombolysis in patients with acute ischaemic stroke (N=1410).
Table 4.
National Institute of Health Stroke Scale (NIHSS) scores changed over time after intravenous thrombolysis
| Outcome | Women (n=541) | Men (n=899) | P value | Adjusted p value* |
| Median NIHSS on admission | 12.3±6.8 | 11.6±6.7 | 0.04 | – |
| Median NIHSS 2 hours (±15 min) | 9.8±7.0 | 9.0±7.2 | 0.004 | – |
| Median NIHSS 24 hours (±2 hour) | 9.1±8.3 | 8.3±7.9 | 0.07 | – |
| Median NIHSS at discharge or 7 days (±1 day) | 6.7±7.8 | 6.2±7.0 | 0.41 | – |
| Improving NIHSS 2 hours (±15 min) | 2.4±4.5 | 2.6±5.0 | 0.66 | 0.59 |
| Improving NIHSS 24 hours (±2 hours) | 3.1±6.7 | 3.2±6.6 | 0.91 | 0.45 |
| Improving NIHSS at discharge or 7 days (±1 day) | 5.2±7.1 | 5.0±6.7 | 0.20 | 0.33 |
*Adjusted baseline variables: age, diastolic blood pressure, current smoking, hypertension, diabetes mellitus, atrial fibrillation, antihypertensive agents before stroke, stroke-to-needle time, stroke-to-visit time, stroke-to-imaging time and Trial of Org 10172 in Acute Stroke Treatment (TOAST) types.
NIHSS, National Institute of Health Stroke Scale.
In terms of safety outcomes, the proportion of SICH and mortality in women is relatively high but did not reach statistical significance (table 2).
Discussion
In this study, we found that the prognosis of intravenous thrombolysis has no significant correlation with sex differences. The trend in the relatively poor prognosis of women may be related to severity and age at the time of onset, rather than sex disparities.
In fact, it has been long recognised that women are more severe and older at the onset of stroke worldwide than men.7 8 25 The reasons for these sex disparities are still not clear. Early studies pointed out that women were more likely to live alone, have atypical symptoms and delay in hospital arrival and acute imaging, potentially resulting in exacerbating the illness.26 27 However, in our study, the functional status before the onset of women and men was similar, and even men took longer to get to the hospital and start thrombolysis than women in the acute phase. This situation may account for the fact that women pay more attention to themselves and have a better understanding of stroke signs and symptoms. Thus, it is of considerable significance to strengthen the education of stroke prevention and treatment to the public.
The influence of sex differences in stroke is also related to age. Recent studies proved that the age-related ischaemic stroke (IS) mortality rate of young women is lower than that of men, while of old women is higher than that of men. But the vital cross-over age is various in different studies.9 16 28 Preclinical research proved that the prognosis of female mice after middle cerebral artery occlusion was even worse with ageing.29 However, in the real world, many studies, including ours, have not found a significant difference in outcomes after thrombolysis between women and men concerning age.16 We believe that the relationship between sex, age and prognosis is not a simple triangle. In fact, a randomised clinical trial named Early versus Late Intervention Trial with Estradiol found out that starting hormone therapy (HT) in newly postmenopausal women (<6 years) is relatively safe and may slow down the progression of atherosclerosis, whereas for women after 10 years of menopause, the effect of HT is not significant.30 More basic research also provides evidence that the impact of age on sex is not only in hormonal changes but also in systemic inflammation, metabolism and age-related gene expression.31–33
Further, our study found that women may benefit more from treatment than men. These observations may be closely tied to the prevalence of cardioembolic stroke, less smoking and better compliance in women. Besides, according to the previous reports, oestrogen plays a crucial role in promoting thrombus recanalisation by lowering the level of platelet activator inhibitors.34
Excluding the sociological and physiological factors above, biological sex differences in genetic are particularly noteworthy when confirmed by various evidence. The most convincing results are that sex-specific cell grown in culture media without sex steroids has a mechanistically different response to hypoxia and glucose deficiency, which is a common manifestation of cerebral ischaemia.35 Moreover, recent work showed an inherent sex disparity in molecular signalling pathways and cell death mechanisms.36
This study has several limitations. First, since it was an observational retrospective study, there was no control group without thrombolytic therapy. Second, only Chinese patients were recruited in the programme. We are unable to analyse the differences in thrombolytic prognosis between women and men of different races. Third, due to the limited sample data and size, the aetiological analysis in women and men is not detailed enough. Factly, gender may be a modifier for various stroke causes.37
Conclusion
For Chinese patients with IS, although women are older and more severe at the time of onset, the prognosis after intravenous thrombolysis is not significantly different from men. It is remarkable to improve women’s chances of thrombolysis and strengthen the genetics and molecular biology research of sex differences per se.
Footnotes
HZ and WC contributed equally.
Contributors: YW designed the study. HZ, WC and YP collected the data. HZ and WC wrote the manuscript. YS, XM and HL reviewed, edited and approved the final version.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81901177, 81971091), Beijing Hospitals Authority Youth Programme (QML20190501), Ministry of Science and Technology of the People’s Republic of China (2016YFC0901002, 2016YFC0901001, 2017YFC1310901, 2017YFC1310902, 2017YFC1307905, 2018YFC1312903,2018YFC1311700 and 2018YFC1311706), Beijing Municipal Administration of Hospitals (SML20150502), Beijing Municipal Science & Technology Commission (D171100003017002, D151100002015003), National Science and Technology Major Project (2017ZX09304018), Beijing Tiantan Hospital, Capital Medical University (2018-YQN-1, 2020MP01) and Young Elite Scientist Sponsorship Program (2020QNRC001).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. 10.1152/physrev.00021.2015 [DOI] [PubMed] [Google Scholar]
- 2. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008;8:737–44. 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salzano A, Demelo-Rodriguez P, Marra AM, et al. A focused review of gender differences in antithrombotic therapy. Curr Med Chem 2017;24:2576–88. 10.2174/0929867323666161029223512 [DOI] [PubMed] [Google Scholar]
- 4. Bellamy L, Casas J-P, Hingorani AD, et al. Pre-Eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazurek M, Huisman MV, Rothman KJ, et al. Gender differences in antithrombotic treatment for newly diagnosed atrial fibrillation: the GLORIA-AF registry program. Am J Med 2018;131:945–55. 10.1016/j.amjmed.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 6. Lantz M, Sieurin J, Sjölander A, et al. Migraine and risk of stroke: a national population-based twin study. Brain 2017;140:2653–62. 10.1093/brain/awx223 [DOI] [PubMed] [Google Scholar]
- 7. Carcel C, Wang X, Sandset EC, et al. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology 2019;93:e2170–80. 10.1212/WNL.0000000000008615 [DOI] [PubMed] [Google Scholar]
- 8. Weber R, Krogias C, Eyding J, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke 2019;50:3494–502. 10.1161/STROKEAHA.119.026723 [DOI] [PubMed] [Google Scholar]
- 9. Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2014;45:1545–88. 10.1161/01.str.0000442009.06663.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019;18:439–58. 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120:439–48. 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 13. Keselman B, Gdovinová Z, Jatuzis D, et al. Safety and outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke: results from the safe implementation of treatments in stroke Registry and meta-analysis. Stroke 2020;51:876–82. 10.1161/STROKEAHA.119.027071 [DOI] [PubMed] [Google Scholar]
- 14. Savitz SI, Schlaug G, Caplan L, et al. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 2005;36:1447–51. 10.1161/01.STR.0000170647.42126.a8 [DOI] [PubMed] [Google Scholar]
- 15. Hametner C, Kellert L, Ringleb PA. Impact of sex in stroke thrombolysis: a coarsened exact matching study. BMC Neurol 2015;15:10. 10.1186/s12883-015-0262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spaander FH, Zinkstok SM, Baharoglu IM, et al. Sex differences and functional outcome after intravenous thrombolysis. Stroke 2017;48:699–703. 10.1161/STROKEAHA.116.014739 [DOI] [PubMed] [Google Scholar]
- 17. Emberson J, Lees KR, Lyden P, et al. Thrombolysis in acute stroke--authors' reply. Lancet 2015;385:1396–96. 10.1016/S0140-6736(15)60718-8 [DOI] [PubMed] [Google Scholar]
- 18. Liao X-L, Wang C-X, Wang Y-L, et al. Implementation and outcome of thrombolysis with alteplase 3 to 4.5 H after acute stroke in Chinese patients. CNS Neurosci Ther 2013;19:43–7. 10.1111/cns.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liao X, Wang Y, Pan Y, et al. Standard-Dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke 2014;45:2354–8. 10.1161/STROKEAHA.114.005989 [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Yang Y, Pan Y, et al. Validation of the simplified Stroke-Thrombolytic predictive instrument to predict functional outcomes in Chinese patients. Stroke 2018;49:2773–6. 10.1161/STROKEAHA.118.022269 [DOI] [PubMed] [Google Scholar]
- 21. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of ORG 10172 in acute stroke treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 22. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in Stroke-Monitoring study (SITS-MOST): an observational study. Lancet 2007;369:275–82. 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 23. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–8. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 24. Larrue V, von Kummer R R, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke 2001;32:438–41. 10.1161/01.str.32.2.438 [DOI] [PubMed] [Google Scholar]
- 25. Bushnell C, Howard VJ, Lisabeth L, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol 2018;17:641–50. 10.1016/S1474-4422(18)30201-1 [DOI] [PubMed] [Google Scholar]
- 26. Bushnell CD, Chaturvedi S, Gage KR, et al. Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab 2018;38:2179–91. 10.1177/0271678X18793324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glader E-L, Stegmayr B, Norrving B, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke 2003;34:1970–5. 10.1161/01.STR.0000083534.81284.C5 [DOI] [PubMed] [Google Scholar]
- 28. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–26. 10.1016/S1474-4422(08)70193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manwani B, Liu F, Scranton V, et al. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 2013;249:120–31. 10.1016/j.expneurol.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the early versus late intervention trial with estradiol testing the menopausal hormone timing hypothesis. Menopause 2015;22:391–401. 10.1097/GME.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res 2017;95:681–91. 10.1002/jnr.23855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaignard P, Fréchou M, Liere P, et al. Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol 2018;30:e12497. 10.1111/jne.12497 [DOI] [PubMed] [Google Scholar]
- 33. Dotson AL, Offner H. Sex differences in the immune response to experimental stroke: implications for translational research. J Neurosci Res 2017;95:437–46. 10.1002/jnr.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kain K, Carter AM, Bamford JM, et al. Gender differences in coagulation and fibrinolysis in white subjects with acute ischemic stroke. J Thromb Haemost 2003;1:390–2. 10.1046/j.1538-7836.2003.00040.x [DOI] [PubMed] [Google Scholar]
- 35. Li H, Pin S, Zeng Z, et al. Sex differences in cell death. Ann Neurol 2005;58:317–21. 10.1002/ana.20538 [DOI] [PubMed] [Google Scholar]
- 36. Liu F, Li Z, Li J, et al. Sex differences in caspase activation after stroke. Stroke 2009;40:1842–8. 10.1161/STROKEAHA.108.538686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen PB, Skjøth F, Overvad TF, et al. Female Sex Is a Risk Modifier Rather Than a Risk Factor for Stroke in Atrial Fibrillation: Should We Use a CHA2DS2-VA Score Rather Than CHA2DS2-VASc? Circulation 2018;137:832–40. 10.1161/CIRCULATIONAHA.117.029081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000351supp001.pdf (90.5KB, pdf)