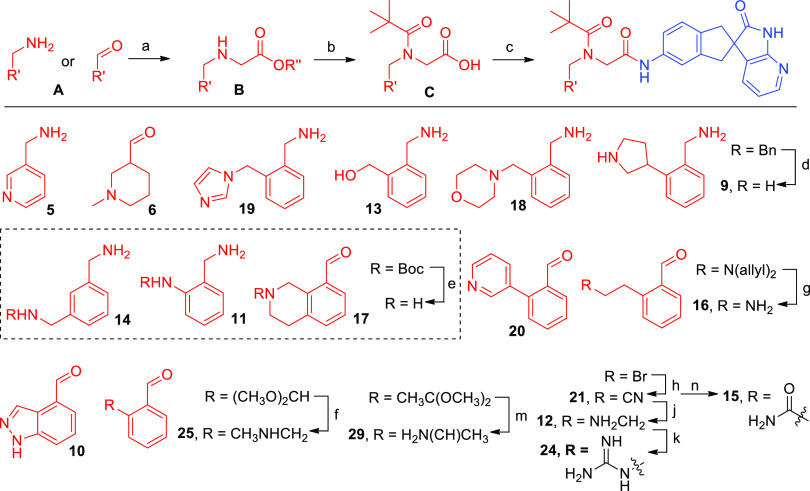
Scheme 4. Synthesis of 5, 6, 9–14, 16–21, 24, 25, and 29.
(a) Ethyl bromoacetate, SIPEA, DMF, rt or benzyl bromoacetate, Et3N, THF, rt (from amine) or glycine ethyl ester hydrochloride, NaBH3CN, MeOH, rt (fromaldehyde or ketone); (b) (i) PivCl, DIPEA, DCM, rt; (ii) 2.5 N NaOH, MeOH, rt; (c) D, HATU, NMM, DMF, rt or D, EDCl, HOAt, DIPEA, DMF, rt; (d) H2, Pd/C, NH4COOH, MeOH, reflux; (e) TFA, DCM, rt or TsOH, MeOH, rt; (f) (i) pTsOH, acetone, rt; (ii) MeNH2, HCl, DIPEA, Na2SO4, DCM, rt then NaBH(OAc)3, rt; (g) 20% Pd(PPh3)4, 1,3-dimethylbarbituric acid, DCM, 35 °C; (h) Zn(CN)2, Pd(PPh3)4, DMF, 130 °C, MW; (j) H2, Raney-Ni, 2M NH3 in MeOH, 55 °C; (k) 4-benzyl-3,5-dimethyl-1H-pyrazole-1-carboximidamide hydrochloride, 5 equiv, Et3N, MeCN/THF, MW, 90 °C; (m) (i) pTsOH, acetone, rt; (ii) NH4OAc, MeOH, reflux; then NaBH3CN, rt; (n) H2O2, H2O, NaOH, DMSO, rt.