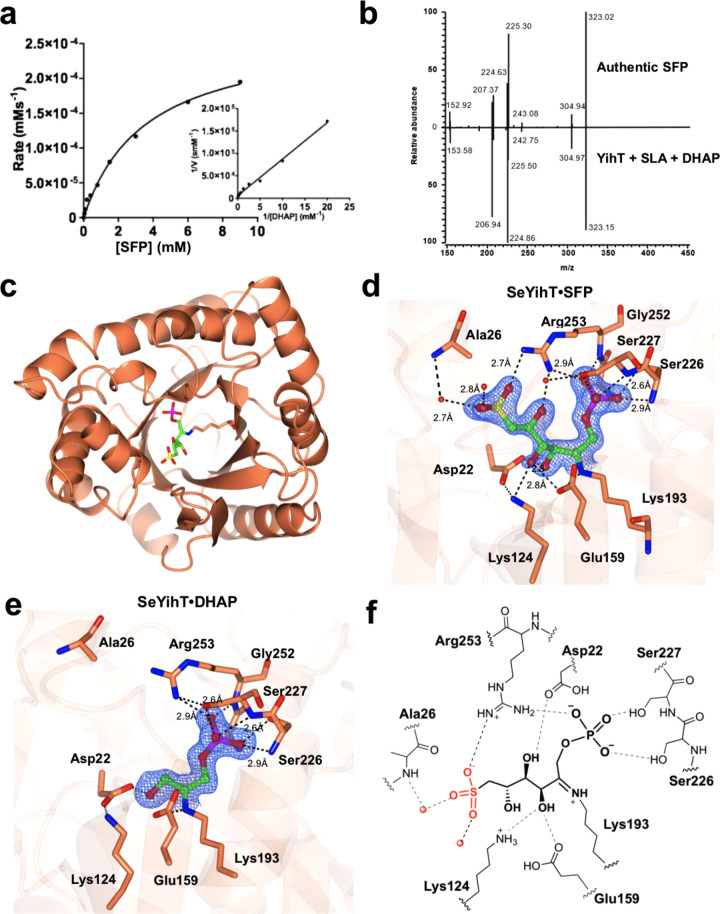
Figure 5.
Kinetics, reversibility, and structure of SeYihT SFP aldolase. (a) Michaelis–Menten and Lineweaver–Burk plots (inset) for SeYihT-catalyzed conversion of SFP to SLA and DHAP, analyzed for DHAP. (b) Mass spectrum mirror plot comparison of the product ion scans of the product of the reaction of SeYihT incubated with SLA and DHAP, and independently synthesized SFP. (c) Overview of the SeYihT protein showing location of substrate binding site. (d) Close-up view of SeYihT·SFP active site with Lys193 engaged in a Schiff base with the sulfosugar. Electron density in blue corresponds to 2Fo – Fc contoured at 1.2σ. (e) Close-up view of SeYihT·DHAP active site with Lys193 engaged in a Schiff base with DHAP. Electron density in blue corresponds to 2Fo – Fc map contoured at 1σ. (f) Cartoon of ligand binding pocket of SeYihT·SFP complex depicting hydrogen bonding and electostatic interactions with active site residues.