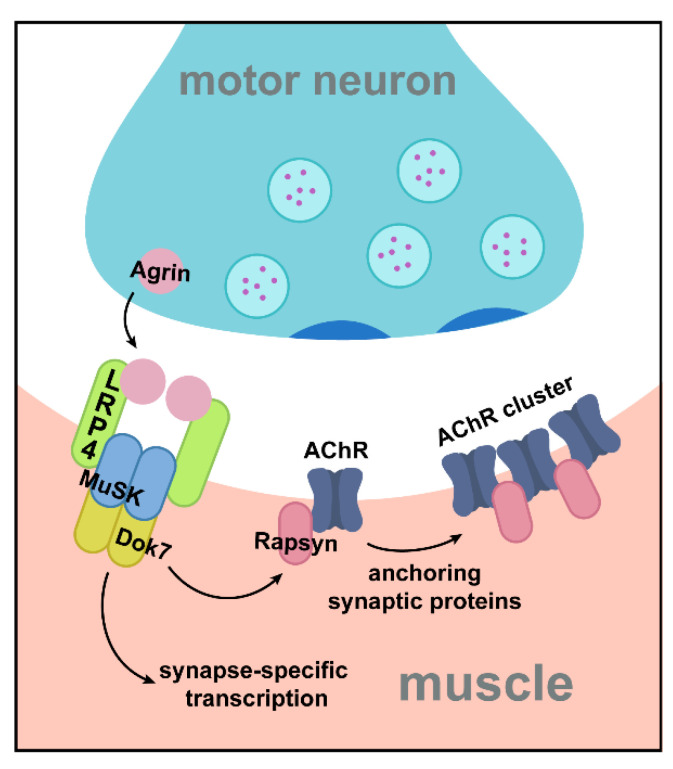
Figure 2.
LRP4 is essential for postsynaptic differentiation at the mammalian NMJ. In its most well-known role at the mammalian NMJ, LRP4 serves as the receptor of Agrin. Agrin is a secreted proteoglycan, derived from the motor neuron, that traverses the synaptic cleft before binding to postsynaptic LRP4. Binding of Agrin to LRP4 induces the formation of a tetrameric complex (containing two molecules of Agrin and two molecules of LRP4) that promotes association with and activation of the receptor tyrosine kinase MuSK. Dimerization of MuSK subsequently signals downstream through Dok7 to instruct aspects of postsynaptic differentiation, including the anchoring of proteins like AChR via interaction with Rapsyn, and transcription of genes encoding synaptic proteins at subsynaptic nuclei.