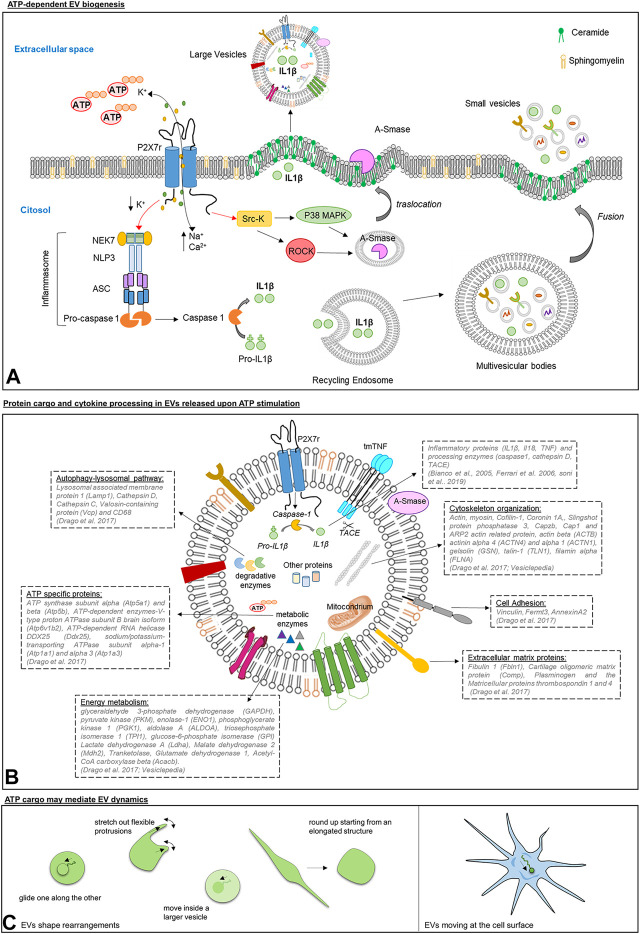
FIGURE 1.
Panel (A): Scheme of ATP/P2X7R signaling axis inducing EV shedding from immune cells. Upon ATP stimulation, P2X7R activates p38 MAPK and ROCK through Src kinases. In turn, p38 and ROCK trigger the local disassembly of the cytoskeletal elements and the mobilization of A-SMASE from the luminal lysosomal compartment to the outer leaflet of the PM, where the enzyme hydrolyzes sphingomyelin to ceramide favoring blebs formation and the shedding of large vesicles carrying IL-1β (Bianco et al., 2005).P2X7R also regulates the release of small EVs. ATP-induced P2X7R stimulation drives the assembly and the activation of the inflammasome composed by regulatory proteins, NEK7, ASC and NLRP3, which are essential for caspase-1 activation. Caspase 1 is a protease implicated in IL-1β processing and in regulating the membrane trafficking pathways that control multivesicular bodies fusion with the PM and the release of IL-1β storing small EVs (Qu et al., 2009). Panel (B): Schematic representation of protein cargo and cytokine processing in large EVs released upon ATP stimulation. By activating P2X7R, ATP induces in large EVs the processing of inflammatory cytokines (Hide et al., 2000; Bianco et al., 2005; Ferrari et al., 2006) and sorting of proteins implicated in autophagy-lysosomal pathway, phagocytosis and endocytosis, energy metabolism and cell adhesion/extracellular matrix organization (Drago et al., 2017). Panel (C): Graphic representation of morphological changes of EVs isolated from human mast cell lines, human blood serum, mouse lung, and Saccharomyces cerevisiae as imaged by Cvjetkovic and colleagues (Cvjetkovic et al., 2017) (left), and of a single EV in motion at the cell surface of microglia (Prada et al., 2016) (right).