Abstract
Background
Plasmodium ovale is an understudied malaria species prevalent throughout much of sub-Saharan Africa. Little is known about the distribution of ovale malaria and risk factors for infection in areas of high malaria endemicity.
Methods
Using the 2013 Democratic Republic of the Congo (DRC) Demographic and Health Survey, we conducted a risk factor analysis for P. ovale infections. We evaluated geographic clustering of infections and speciated to P. ovale curtisi and P. ovale wallikeri through deep sequencing.
Results
Of 18 149 adults tested, we detected 143 prevalent P. ovale infections (prevalence estimate 0.8%; 95% confidence interval [CI], .59%–.98%). Prevalence ratios (PR) for significant risk factors were: male sex PR = 2.12 (95% CI, 1.38–3.26), coprevalent P. falciparum PR = 3.52 (95% CI, 2.06–5.99), and rural residence PR = 2.19 (95% CI, 1.31–3.66). P. ovale was broadly distributed throughout the DRC; an elevated cluster of infections was detected in the south-central region. Speciation revealed P. ovale curtisi and P. ovale wallikeri circulating throughout the country.
Conclusions
P. ovale persists broadly in the DRC, a high malaria burden country. For successful elimination of all malaria species, P. ovale needs to be on the radar of malaria control programs.
Keywords: Plasmodium ovale, nonfalciparum malaria, epidemiology, amplicon sequencing
Plasmodium ovale is present and broadly distributed throughout the Democratic Republic of Congo (DCR) at an estimated prevalence of 0.8%. Both species, P. ovale wallikeri and P. ovale curtisi, were identified as occurring throughout the DRC.
There are 6 species of the malaria parasite known to infect humans, 2 of which, Plasmodium ovale wallikeri and Plasmodium ovale curtisi, form a species complex known as Plasmodium ovale [1]. One of the most ancestral of the human infecting Plasmodium spp. [2, 3], the geographic distribution of P. ovale is considered broad and thought to encompass most of the African continent and much of Southeastern Asia [1, 4–6]. Despite this wide geographic distribution, ovale malaria is a neglected disease with few epidemiological studies investigating the prevalence and associated risk factors. The neglected status of P. ovale is likely due to multiple factors: (1) ovale malaria typically has a mild, and often asymptomatic, clinical course [1, 5, 6]; (2) P. ovale parasite densities are generally lower compared with other Plasmodium spp. [5–8]; (3) P. ovale is often detected as a mixed species infection in areas with high malaria transmission intensity [1, 4–6, 8]. The culmination of low parasite counts and mixed infections yields scenarios where misdiagnosis of ovale malaria is common [4–6], hampering accurate estimates of P. ovale in areas with high prevalence of malaria. This issue of misdiagnosis and the lack of epidemiologic data on the geographic range and corresponding prevalence of P. ovale in endemic zones should be of particular concern in an era of rigorous malaria elimination efforts.
The Democratic Republic of the Congo (DRC) has the second highest burden of malaria in the world [9, 10]. Previous studies have shown that P. falciparum, P. vivax, P. malariae, and P. ovale all circulate in the DRC [9, 11], and many studies have focused extensively on investigating risk factors associated with P. falciparum infection to inform elimination interventions [9, 11, 12]. However, few studies have examined the distribution of P. ovale in this zone of high malaria transmission intensity [9, 11] and none have identified risk factors associated with infection. Additionally, the geographic location of the DRC is important for malaria epidemiology in Africa given it appears to be a bridge between East and West African parasite populations and therefore is likely a microcosm of the region [13].
We evaluated the distribution of prevalent ovale malaria in the DRC by using real-time polymerase chain reaction (PCR) to detect parasites from blood spots collected from the 2013–2014 Demographic and Health Survey (DHS), a nationally population-representative survey. Unlike previous studies, we were able to robustly assess for risk factors associated with P. ovale infection and evaluate spatial clustering of prevalent infections in the DRC. Using these data, which represent the largest, nationally representative study of P. ovale to date, we showed that P. ovale is broadly prevalent in the DRC and continues to be circulating at relatively high frequencies, consistent with estimates across the sub-Saharan African region. For successful elimination of malaria, knowledge of the epidemiology of all species of malaria is essential.
METHODS
The 2013–2014 DRC DHS was a cross-sectional study that collected demographic, social, and population health data on a nationally representative sample of adults in the DRC between August of 2013 and February of 2014. The survey followed a 2-stage cluster design composed of 540 sampling clusters representative of the 26 designated DRC provinces. Interviews were conducted for selected households within each sampling cluster. A total of 18 257 dried blood spot (DBS) samples were collected from adults, aged 15–59 for men and 15–49 for women, to test for HIV prevalence. HIV testing was conducted in Kinshasa, after which all DBS samples were transported to the University of North Carolina at Chapel Hill for molecular analysis of malaria infections. DBS samples had DNA extracted using Chelex methods and underwent testing for malaria [14]. The study design and procedures were approved by the institutional review board of the University of North Carolina at Chapel Hill (number 19-0573).
Detection of the P. ovale complex was conducted by real-time PCR modified from previously published methods [15]. All PCRs were run with nontemplate controls and a serial dilution of 18S P. ovale plasmid (MRA-180; BEI Resources) to help estimate parasite density. The plasmid was derived from a P. ovale curtisi isolate with a few mismatches to P. ovale wallikeri sequences. Our PCR assay included variable nucleotides for positions differing between the 2 species. The number of genomes for each serial dilution was estimated assuming 6 plasmid copies per genome equivalent for P. ovale [16].
Further speciation to P. ovale wallikeri and P. ovale curtisi, was conducted for all PCR-positive samples using amplicon deep sequencing methods [17] that adapted a previously described speciation real-time PCR assay [18]. All samples were amplified and sequenced alongside appropriate nontemplate and positive controls. Details of amplicon sequencing are provided in the Supplementary Material. Haplotypes based on the amplified region of the 18S ribosomal subunit gene were determined using SeekDeep [19]. Following previous approaches for single replicates, haplotypes with a frequency of greater than 0.05% were included [20] (Supplementary Material).
A point prevalence of the P. ovale species complex based on real-time PCR was the primary outcome in this study. Additional variables of interest included age, sex, infection with P. falciparum, urban or rural residence, wealth, long-lasting insecticidal net (LLIN) use, household livestock ownership, education, and anemia. Infection with P. falciparum was detected by PCR in concordance with this study but published elsewhere [21]. All other covariates were derived from the DHS household survey. The functional form of age was assessed by a LOESS (locally estimated scatterplot smoothing) line of ovale malaria cases by age. All ages greater than 45 years were collapsed at 45 to prevent skewed estimates among sparse older age groups. Wealth status was dichotomized as poor or nonpoor, with nonpoor including all middle, richer, and richest wealth categories as defined by the DHS. Education was collapsed categorized as none, primary school only, and secondary school or higher. Anemia was categorized as moderate to severe, mild, or not anemic. To correct for the sampling strategy, DHS defined weights were applied to our prevalence estimates and used in models according to DHS weighting instructions [22].
We used binomial logarithmic generalized estimating equations (GEE) to account for correlation within sampling clusters and estimate crude prevalence estimates as well as effect estimates with 95% confidence intervals (CIs) for our covariates of interest. Prevalence ratio estimates for sex, P. falciparum infection, rural residence, bed-net use, and household livestock ownership were adjusted for in a multivariable log-binomial model with the addition of age as a restricted quadratic term. Collinearity between covariates of interest was tested for using a correlation matrix of the covariates. Any covariate with a correlation coefficient of 0.5 or greater was eliminated from the final adjustment set. Final model fit was evaluated based on substantive knowledge of ovale malaria predictors and Akaike information criterion values. Statistical significance for prevalence ratios (PR) was defined as exclusion of the null (PR = 1) in the 95% CI. All models were run in R (Version 3.5.1). DHS survey weights were applied using the survey package [23] and used for all consecutive analyses.
The spatial distribution of P. ovale infections in the DRC was mapped as percent prevalence aggregated at the province level and weighted according to DHS weighting instructions, as described above. Global positioning system (GPS) coordinates were available for 482 of the 540 DHS sampling clusters and were offset by 2 km in urban areas and 10 km in rural areas to protect participant privacy. We assumed that coordinate missingness is noninformative and did not adjust for it in this analysis. Spatial clustering among the survey clusters was evaluated using SaTScan software (version 9.6) [24] assuming a Poisson distribution of P. ovale infections among the study sample. We tested for both high and low spatial clusters with .05 as our significance threshold. Only clusters of high P. ovale prevalence considered significantly different from surrounding P. ovale prevalence levels are reported here. DRC cities with an estimated population greater than 250 000 people in 2000 from the Humanitarian Data Exchange were included for reference [25]. Final map figures were generated in ArcMap (version 10.5).
To compare our results to neighboring countries and for a more expansive understanding of the distribution of P. ovale across the African region, a literature search was conducted to capture the most recent, population-based, prevalence estimates of P. ovale infection among sub-Saharan African nations. Both PubMed and Google Scholar were queried using the terms: Plasmodium ovale, prevalence, and the country of interest. Studies were excluded if they only described imported infections or were case reports. Additional information summarized from each study included: asymptomatic or symptomatic infection, presence of other infecting malaria species (mixed or monoclonal), method of detection, study design and population, and study year.
RESULTS
The P. ovale prevalence estimates reported in the literature for sub-Saharan Africa (Table 1) ranged from 0.2% population prevalence in Angola to 4.8% population prevalence in Malawi (including mixed infections). For monoclonal infections, prevalence estimates ranged from 0.2% clinical cohort prevalence in the Central African Republic to 4.2% population prevalence in Cameroon. Most infections were asymptomatic and in 13 of the 16 studies, P. ovale was detected only as a mixed infection with P. falciparum.
Table 1.
Recent Prevalence Estimates for Plasmodium ovale Among sub-Saharan African Countries Based on Previously Published Studies
| Country | Number of Ovale Cases/Study Sample | Prevalence, % | Symptomatic/ Asymptomatic | Mixed Infection or Monoclonal | Detection Method | Study Type | Study Yeara | Population | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Central Africa | |||||||||
| Democratic Republic of Congo | 153/17 765 | 0.8 | Asymptomatic | Mixed | PCR | CS surveyb | 2013–2014 | Adults, 15+ y | This study |
| Angola | 23/3316 | 0.2 | Asymptomatic | Mixed | PCR | CS surveyb | 2010 | All ages | [26] |
| Cameroon | 31/742 | 4.2 | Asymptomatic | Monoclonal | Immunofluorescence | Surveyb | 1983 | All ages | [27] |
| Central African Republic | 1/437 | 0.2 | Symptomatic | Monoclonal | Microscopy, PCR | Clinical | 2011 | Febrile patients, all ages | [28] |
| Gabon | 4/206 | 1.9 | Symptomatic | Mixed | PCR | Clinical | 2005 | All ages | [29] |
| Republic of Congo | 11/851 | 1.3 | Symptomatic | Mixed | PCR | Clinical, passive case detection | 2005 | All ages | [29] |
| East Africa | |||||||||
| Tanzania | 21/511 | 4.1 | Asymptomatic | Mixed | PCR | CS surveyb | 2016 | All ages | [30] |
| Zambia | 18/873 | 2.1 | Asymptomatic | Mixed | PCR | CS surveyb | 2012 | All ages | [31] |
| Malawi | 138/2873 | 4.8 | Asymptomatic | Mixed | PCR | CS surveyb | 2002 | All ages | [32] |
| Southern Sudan | 1/392 | 0.3 | Symptomatic | Mixed | Microscopy | CS surveyb | 1975 | All ages | [33] |
| Uganda | 36/2108 | 1.7 | Asymptomatic | Mixed | PCR | Clinical, CS survey | 2009 | All ages | [7] |
| Kenya | 35/722 | 4.8 | Symptomatic | Mixed | PCR | Clinical, active case detection | 1998 | All ages | [29] |
| West Africa | |||||||||
| Ghana | 4/270 | 1.5 | Asymptomatic | Monoclonal | PCR | Longitudinalb | 2010 | Children 5–17 years | [34] |
| Nigeria | 126/5211 | 2.4 | Asymptomatic | Mixed | Microscopy | CS surveyb | 2010 | Children < 5 years | [35] |
| Liberia | 19/434 | 4.4 | Asymptomatic | Mixed | Microscopy | CS surveyb | 1957 | All ages | [36] |
| Senegalc | 719/29 280 | 2.5 | Asymptomatic | Mixed | Microscopy | CS survey, longitudinalb | 1990–2010 | All ages | [37] |
Abbreviations: CS, cross-sectional, PCR, polymerase-chain reaction.
aYear(s) of sample collection.
bStudy is representative of the sampled population (ie, symptomatic based sampling methods were not employed).
cLongitudinal cross-sectional sampling of 1 study site across 20 years.
PCR screening for P. ovale was completed for a total of 17 765 DBS representing adults aged 15 to 59 years in the DRC between 2013 and 2014. A total of 492 DBS were unable to be screened due to contamination during shipping or insufficient sample quality; reasons for exclusion of DBS are summarized in Supplementary Figure 1. Among the screened samples, P. ovale DNA was detected in 153 samples for a crude period prevalence estimate of 0.86%. The P. ovale-positive samples had a median parasite density of 14 parasites/μL with a range of 0.64–942 parasites/μL. The sampling structure across the full study population was adjusted for using DHS administered sampling weights, providing a final estimate of 143 prevalent P. ovale infections (95% CI, 105–180) among 18 149 individuals (95% CI, 16 549–19 749) for a nationally weighted prevalence estimate of 0.79% (.59%–.98%). Henceforth, all estimates and models were conducted using survey weights. Among all P. ovale-positive individuals, overall median age was 24 years (interquartile range [IQR], 17–37), among men it was 25 years (IQR, 18–43), and 20 years (IQR, 15–25) for women. A majority of positive individuals were male (66%), positive for P. falciparum infection (62%), resided in a rural area (79%), reported LLIN use (54%), and lived in a household that owned livestock (66%) (Table 2).
Table 2.
Characteristics of Adult Respondents in the 2013–2014 Demographic and Health Survey in the Democratic Republic of Congo, Overall and by Plasmodium ovale Infection Status
| Characteristics | Total | P. ovale Status | |
|---|---|---|---|
| Yes | No | ||
| n | 18 149 | 143 | 18 006 |
| Age, y, median (IQR) | 28 (20–38) | 24 (17–37) | 28 (20–38) |
| Male | 8619 (47.5) | 94 (65.7) | 8525 (47.3) |
| P. falciparum infection | 5646 (31.1) | 88 (61.5) | 5558 (30.9) |
| Rural | 11 551 (63.6) | 113 (79.0) | 11 438 (63.5) |
| Poor | 6836 (37.7) | 61 (42.7) | 6775 (37.6) |
| LLIN use | 9682 (53.3) | 77 (53.8) | 9605 (53.3) |
| Livestock ownership | 8938 (49.2) | 95 (66.4) | 8843 (49.1) |
| Education | |||
| No education | 1905 (10.5) | 4 (2.8) | 1901 (10.6) |
| Primary | 5615 (30.9) | 47 (32.9) | 5568 (30.9) |
| Secondary | 10 612 (58.5) | 92 (64.3) | 10 520 (58.4) |
| Missing | 18 (0.1) | 0 (0) | 18 (0.1) |
| Anemia | |||
| Moderate-severe | 1020 (5.6) | 13 (9.1) | 1007 (5.6) |
| Mild | 3540 (19.5) | 39 (27.3) | 3501 (19.4) |
| Not anemic | 13 541 (74.6) | 91 (63.6) | 13 450 (74.7) |
| Missing | 48 (0.3) | 0 (0) | 48 (0.3) |
Data are No. (%) except where indicated.
Abbreviations: IQR, interquartile range; LLIN, long-lasting insecticidal net.
Risk Factor Analysis
Univariate GEE log-binomial regressions identified male sex, coprevalent P. falciparum infection, rural household residence, and household livestock ownership as significantly associated with an increase in ovale prevalence compared with referent levels of each variable. Based on these estimates, ovale prevalence among men was 2.12 that of women (95% CI, 1.38–3.26); among individuals with PCR-confirmed P. falciparum infection, it was 3.52 times that of those negative for P. falciparum (95% CI, 2.06–5.99); among rural residents it was 2.19 times that of their urban counterparts (95% CI, 1.31–3.66); and among those owning livestock, it was 2.07 that of participants reporting no livestock ownership (95% CI, 1.27–3.37). Bed-net use (PR, 1.02; 95% CI, .63–1.62) and wealth (PR, 1.25; 95% CI, .76–2.06) were not significantly associated with a relative difference in ovale malaria prevalence among participants.
To account for confounding bias, we used multivariate GEE log binomial regression models. Collinearity between wealth and urbanicity was detected with a r2 value of 0.46, therefore wealth was removed from the final adjustment set. Our final multivariable model included age as a restricted term and a restricted quadratic term, sex, coprevalent P. falciparum infection, rurality, bed-net use, and livestock ownership. Following adjustment, all relative prevalence estimates were shifted down and slightly closer to the null, with the exception of bed-net use, which was shifted up and away from the null. All adjusted estimates are summarized along with crude estimates in Table 3.
Table 3.
Crude and Multivariate Adjusted Prevalence and Prevalence Ratio Estimates of Plasmodium ovale Infections Among Congolese Adults in the Democratic Republic of Congo for 2013
| Crude Prevalence Ratio | Adjusted Prevalence Ratioa | |||||
|---|---|---|---|---|---|---|
| Covariate | Prevalence, % | 95% CI | PR | 95% CI | PR | 95% CI |
| Sex | ||||||
| Female | 0.51 | (0.35–0.74) | Ref. | Ref. | ||
| Male | 1.09 | (0.82–1.45) | 2.12 | (1.38–3.26) | 1.89 | (1.25–2.87) |
| P. falciparum infection | ||||||
| No | 0.44 | (0.28–0.69) | Ref. | Ref. | ||
| Yes | 1.55 | (1.19–2.03) | 3.52 | (2.06–5.99) | 2.92 | (1.66–5.12) |
| Urban/rural | ||||||
| Urban | 0.45 | (0.29–0.69) | Ref. | Ref. | ||
| Rural | 0.98 | (0.74–1.30) | 2.19 | (1.31–3.66) | 1.78 | (1.03–3.07) |
| LLIN use | ||||||
| No | 0.78 | (0.57–1.07) | Ref. | Ref. | ||
| Yes | 0.79 | (0.56–1.13) | 1.02 | (0.63–1.62) | 1.14 | (0.73–1.79) |
| Livestock ownership | ||||||
| No | 0.52 | (0.34–0.79) | Ref. | Ref. | ||
| Yes | 1.07 | (0.80–1.41) | 2.07 | (1.27–3.37) | 1.58 | (0.93–2.67) |
| Wealth | ||||||
| Nonpoor | 0.72 | (0.50–1.03) | Ref. | … | ||
| Poor | 0.90 | (0.64–1.26) | 1.25 | (0.76–2.06) | … | … |
Abbreviations: CI, confidence interval; LLIN, long-lasting insecticidal net; PR, prevalence ratio; Ref., reference level.
aAdjusted for age modelled as a restricted quadratic term, male sex, P. falciparum infection, rural residence, LLIN use, and household livestock ownership.
Geographic Distribution of Ovale Malaria in the DRC
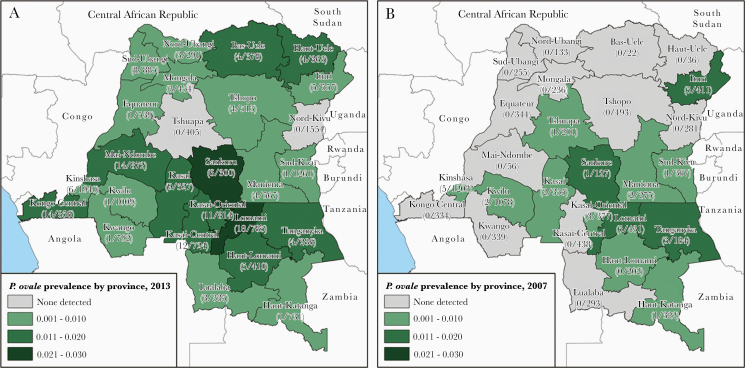
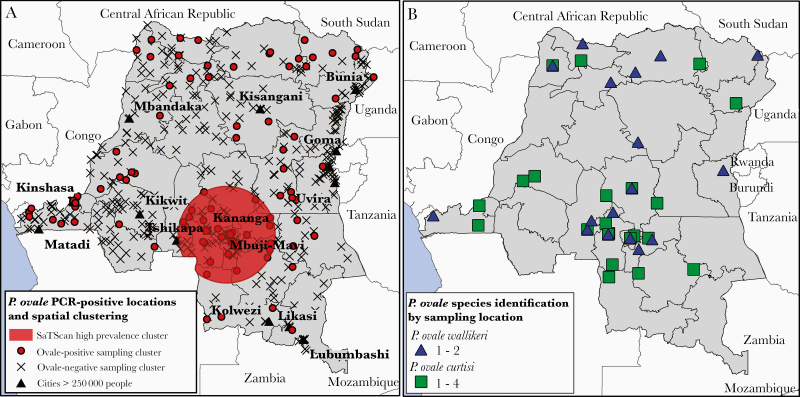
Prevalent ovale malaria was detected in 24 of the 26 DRC provinces. Prevalence estimates ranged from a maximum of 2.3% prevalence in Lomami province to a minimum of 0.01% prevalence in Sud-Kivu province (Figure 1A). Areas of higher P. ovale prevalence were concentrated in western, south-central, and north-eastern areas of the county. The 2 highest prevalence provinces, Lomami and Sankuru, located in the south-central area of the country also exhibited high prevalence in the 2007 DHS survey (Figure 1B). SaTScan identified 1 significantly distinct spatial cluster of high prevalence ovale malaria among study sampling clusters (P = .0014) in south-central DRC. The radius of the high spatial cluster was 283 km, and it fully encompassed the Kasai-Central, Kasai-Oriental, and Lomami provinces, as well as regions in the Kasai, Sankuru, Maniema, Haut-Lomami, and Lualaba provinces (Figure 2A).
Figure 1.
Point prevalence estimates of Plasmodium ovale infections by Democratic Republic of the Congo province (A) for the 2013 Demographic and Health Survey (DHS) of 18 149 adults and (B) for the 2007 DHS survey of 8793 adults. The number of positive ovale samples and the province sample size (weighted) is listed below each province name.
Figure 2.
A, Spatial clustering of ovale malaria infections detected by SaTScan. Large cities with a population estimate of greater than 250 000 in 2000 and Demographic and Health Survey sampling cluster locations are included for reference. Sample clusters represented by a closed circle indicate the presence of 1 or more individuals positive for Plasmodium ovale, clusters represented with an “X” indicate that no P. ovale was detected. B, Sampling locations corresponding to P. ovale curtisi and P. ovale wallikeri-positive samples. For 12 samples across 5 provinces, global positioning system (GPS) sampling cluster coordinates were missing; these samples were plotted at the centroid coordinates of their respective province.
Speciation to P. ovale wallikeri and P. ovale curtisi
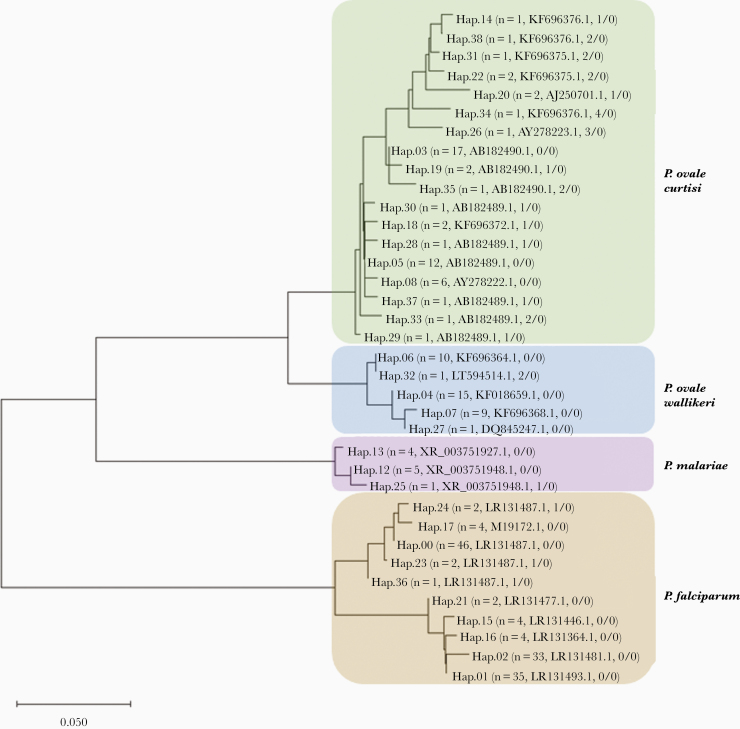
Of the 153 samples that were PCR-positive for P. ovale, 62 were of sufficient quality and quantity for the detection of distinct Plasmodium spp. 18S haplotypes. A total of 35 haplotypes were successfully identified across the 62 samples. Twenty-three of them aligned with reported P. ovale species in GenBank: 18 with P. ovale curtisi and 5 with P. ovale wallikeri. Ten haplotypes of P. falciparum and 3 haplotypes of P. malariae were also found. Genetic relatedness across all haplotypes was assessed with MEGA version X [38] using a neighbor-joining tree (Figure 3). Based on our haplotype analysis, 23 samples (37%) were positive for P. ovale curtisi, 16 (26%) were positive for P. ovale wallikeri, 3 (5%) contained both P. ovale curtisi and P. ovale wallikeri, and 20 (32%) were identified as P. falciparum or P. malariae with no detectable P. ovale spp. haplotypes in the SeekDeep analysis. Of these 20 samples, 15 had reads that mapped to 1 of the P. ovale spp. using a k-mer alignment approach, but likely remained undetected due to filtering cutoffs for quality assurance [39, 40]. The 5 remaining samples had a median parasite density of 3 parasites/μL according to real-time PCR results and were likely missed by sequencing analysis algorithms, detecting only the coinfecting falciparum species.
Figure 3.
Neighbor-joining tree of genetic relatedness across the 35 haplotypes identified from pooled amplicon deep sequencing results of 62 Plasmodium ovale polymerase chain reaction (PCR)-positive samples. Each unique haplotype is numbered and branches of the tree for each malaria species are laid over a color (brown, P. falciparum; purple, P. malariae; blue, P. ovale wallikeri; and green, P. ovale curtisi). In parentheses after the haplotype number is the number of samples that contained the haplotype, the GenBank accession name for closest match, and the number of single nucleotide polymorphisms/number of indels difference in the haplotype from the closest GenBank reference. Twenty-three samples (37%) were positive for P. ovale curtisi, 16 (26%) were positive for P. ovale wallikeri, and 3 (5%) contained both P. ovale curtisi and P. ovale wallikeri. However, given that the malaria genome contains multiple copies of the 18S gene, samples may contain more than 1 haplotype of a species. The scale bar represents the number of base substitutions per site.
Samples that were successfully sequenced did not differ significantly from those that were unsuccessful for any of the P. ovale risk factors identified, except for coprevalent P. falciparum infection (Supplementary Table 1). Successfully sequenced samples exhibited a significantly higher median parasite densities compared to the unsuccessful samples (23 vs 9 parasites/μL, P = .007). Characteristics of participants positive for each ovale species did not differ significantly, though individuals positive for P. ovale wallikeri appeared to be slightly older and less likely to report using a bed net than P. ovale curtisi-positive individuals (Supplementary Table 2). Geographically, there appeared to be some separation between the ovale species, but the small sample size precluded geostatistical testing (Figure 2B).
DISCUSSION
We estimate that in the 2013–2014 DHS, 0.79% of Congolese adults (95% CI, .59%–.98%) had a prevalent ovale malaria infection, as detected by real-time PCR. This is close to the prevalence previously reported using the 2007 DHS of 0.6% (95% CI, .1–.9) [9] but substantially lower than many prevalence estimates for surrounding nations (Table 1). Many of the studies reporting higher prevalence estimates targeted malaria survey sites [26, 29–34, 36, 37] or employed clinic-based sampling methods [7, 28] to select for individuals at higher risk for P. falciparum malaria infections. Given the association between infection with P. ovale and coinfection with P. falciparum, this may artificially inflate estimates of P. ovale prevalence in population-targeted studies. In contrast, the DHS was designed to be nationally representative of the DRC and intentionally corrects for oversampling of rural areas, which generally harbor higher levels of malaria, to reduce potential selection bias. Additionally, our study only examined adults, who often have lower and difficult to detect P. ovale parasite densities than children [1, 5], which may also contribute to the lower prevalence estimate.
This study is unique compared to prior studies of ovale prevalence, in that it examined not only the geographic distribution of prevalent infections, but also risk factors associated with P. ovale. We found that ovale malaria is widespread throughout the DRC: 24 of 26 provinces had at least 1 prevalent ovale infection detected (Figure 1A). SaTScan analysis suggested the existence of a single cluster of higher prevalence than expected spanning the Kasai-Central, Kasai-Oriental, and Lomami provinces in south-central DRC (Figure 2A). This cluster area included Mbuji-Mayi, the capital city of Kasai-Oriental and estimated to be the third largest city in the DRC. This same geographic area also showed higher P. ovale prevalence in the previous 2007 DHS screen, suggesting the presence of possible underlying spatial processes supportive of higher, stable ovale malaria transmission (Figure 1B).
Intriguingly, we found that men had twice the prevalence of ovale malaria compared with women in our study sample. The median age of infection among men (25 years), was higher than that of women (20 years), but lower than the overall median age of all study participants regardless of ovale infection (28 years). A potential explanation of this biological-sex and age pattern may be that ovale infections are more likely to occur in men of working age due to work-based exposure. The “hotspot” around Mbuji-Mayi is replete with active gem mines [41] and this may partially account for the excess infections in men. A recent study of P. ovale spp. infection in returning travelers, also reported that men were more likely than women to be infected with P. ovale wallikeri, but not P. ovale curtisi [42]. We found that both species are present in the DRC based on our deep sequencing results; however, any difference in infection frequencies with the 2 species in men versus women was not observed (75% of P. ovale wallikeri infections were among men vs 74% of P. ovale curtisi; Supplementary Table 2). Given the relatively small number of P. ovale-positive samples that were speciated, further studies are needed to elucidate patterns of both ovale species in the DRC and evaluate if heightened prevalence among men holds for both species or only one.
The strongest association with ovale infection was coprevalent P. falciparum infection. Individuals infected with P. falciparum had nearly a 3 times higher average prevalence of ovale infections compared with those who were falciparum negative when adjusting for age, sex, rurality, bed-net use, and livestock ownership (Table 3). This association likely represents exposure to an area with high malaria transmission intensity, further supported by the strong association between ovale prevalence and rural residence—a factor known to be associated with increased P. falciparum prevalence [9, 11, 21] .However, it is important to note that 38% of P. ovale-positive samples were negative for P. falciparum, suggesting that while the ecological niche of both species clearly overlaps, it does not preclude single-species infection. Additionally, we found insufficient evidence for an effect of LLIN use on ovale prevalence. This is in contrast to a protective effect of bed nets on P. falciparum infection, where use in the DRC is associated with an estimated 20% decrease in prevalence [21]. One possible explanation might be due to the hypnozoite life stage that occurs with P. ovale but not P. falciparum, allowing for persistence of ovale parasites and relapse of infection [1, 5]. Unfortunately, treatments required to eliminate hypnozoite stage parasites differ from widely available treatments for P. falciparum infections, possibly allowing for reemergence of ovale parasites following elimination of blood-stage P. falciparum parasites. Thus, concerns might arise over spatial overlap, and separation, between ovale and falciparum malaria with regard to control and elimination strategies.
Further studies will be crucial for confirming if control and elimination strategies targeting P. falciparum are as effective with P. ovale, or if additional strategies will be necessary to also eliminate this broadly distributed and chronically underdetected species of malaria. In addition, while our study provides insight into epidemiological factors associated with P. ovale occurrence in a high burden population, further studies are needed to reevaluate these risk factors among a larger sample of the 2 P. ovale species to fully characterize the extent to which the epidemiological profiles of P. ovale curtisi and P. ovale wallikeri overlap. Additionally, this study only used ribosomal RNA genes to identify and speciate P. ovale. The use of an assay targeting additional nuclear genes might improve specificity in future studies, and potentially enhance detection of low-density infections. These insights are needed to inform malaria elimination campaigns for the reduction of all species of malaria.
This study represents the largest, nationally representative sample assessed for P. ovale (Table 1) and provides the most robust assessment of P. ovale risk factors to date. We found P. ovale malaria infections to be prevalent throughout the DRC and identified male sex and coprevalent P. falciparum infection as significant risk factors contributing to increased prevalence among Congolese adults. Additionally, we identified an area in the south-central DRC significantly associated with increased prevalence based on our 2013 screen, which appears to have harbored a higher proportion of infections based on the 2007 screen as well. Finally, we were able to speciate a subset of P. ovale PCR-positive samples using deep sequencing methods and identified P. ovale curtisi and P. ovale wallikeri infections circulating throughout the DRC as mixed and single-species infections. Our findings provide insight into the epidemiology of this neglected malaria species in one of the most malarious countries in the world.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the contributions of the following people for their insight into this project and analytical methods: Molly Deutsch-Feldman, Jonathan Parr, and Kyaw “Jolly” Thwai. This project would not have been possible without the tireless work of the team who implemented the Demographic and Health Survey in the DRC or the participants who consented to be a part of the survey. All P. ovale plasmid stocks were obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium ovale, MRA-180, contributed by Peter A. Zimmerman.
Disclaimer. The funder had no role in study design, data collection, or interpretation.
Financial support. This work was supported by the National Institutes of Health through the National Institute of Allergy and Infectious Diseases (grant numbers K24AI134990 to J. J. J., R01AI107949 to S. R. M, F30AI143172 to N. F. B.), the Fogarty International Center (R01TW010870 to J. J. J), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32HD007168 to C. K.) and the National Institute of Environmental Health Sciences (T32ES007018 to C. K.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society for Tropical Medicine 68th Annual Meeting, National Harbor, MD, 20–24 November 2019.
References
- 1. Sutherland CJ, Tanomsing N, Nolder D, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis 2010; 201:1544–50. [DOI] [PubMed] [Google Scholar]
- 2. Rutledge GG, Böhme U, Sanders M, et al. Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution. Nature 2017; 542:101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loy DE, Liu W, Li Y, et al. Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int J Parasitol 2017; 47:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ 1969; 40:383–94. [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol 2007; 23:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev 2005; 18:570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oguike MC, Betson M, Burke M, et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol 2011; 41:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanomsing N, Imwong M, Sutherland CJ, et al. Genetic marker suitable for identification and genotyping of Plasmodium ovale curtisi and Plasmodium ovale wallikeri. J Clin Microbiol 2013; 51:4213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor SM, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One 2011; 6:e16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. World malaria report 2019. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 11. Doctor SM, Liu Y, Anderson OG, et al. Low prevalence of Plasmodium malariae and Plasmodium ovale mono-infections among children in the Democratic Republic of the Congo: a population-based, cross-sectional study. Malar J 2016; 15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levitz L, Janko M, Mwandagalirwa K, et al. Effect of individual and community-level bed net usage on malaria prevalence among under-fives in the Democratic Republic of Congo. Malar J 2018; 17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verity R, Aydemir O, Brazeau NF, et al. The impact of antimalarial resistance on the genetic structure of Plasmodium falciparum in the DRC. Nat Commun 2020; 11:2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995; 52:565–8. [DOI] [PubMed] [Google Scholar]
- 15. Srisutham S, Saralamba N, Malleret B, Rénia L, Dondorp AM, Imwong M. Four human Plasmodium species quantification using droplet digital PCR. PLoS One 2017; 12:e0175771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mercereau-Puijalon O, Barale JC, Bischoff E. Three multigene families in Plasmodium parasites: facts and questions. Int J Parasitol 2002; 32:1323–44. [DOI] [PubMed] [Google Scholar]
- 17. Lundberg DS, Yourstone S, Mieczkowski P, Jones CD, Dangl JL. Practical innovations for high-throughput amplicon sequencing. Nat Methods 2013; 10:999–1002. [DOI] [PubMed] [Google Scholar]
- 18. Mangold KA, Manson RU, Koay ES, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 2005; 43:2435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hathaway NJ, Parobek CM, Juliano JJ, Bailey JA. SeekDeep: single-base resolution de novo clustering for amplicon deep sequencing. Nucleic Acids Res 2018; 46:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyce RM, Brazeau N, Fulton T, et al. Prevalence of molecular markers of antimalarial drug resistance across altitudinal transmission zones in highland Western Uganda. Am J Trop Med Hyg 2019; 101:799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deutsch-Feldman M, Brazeau N, Parr J, et al. Spatial and epidemiological drivers of Plasmodium falciparum malaria among adults in the Democratic Republic of the Congo. BMJ Glob Health 2020; 5:e002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Croft T, Aileen M, Courtney A Guide to DHS statistics. Rockville, MD: ICF, 2018. [Google Scholar]
- 23. Lumley T. Analysis of complex survey samples. J Stat Softw 2004; 9: doi: 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- 24. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods 1997; 26:1481–96. [Google Scholar]
- 25. Humanitarian Data Exchange. Democratic Republic of the Congo–major cities.https://data.humdata.org/dataset/democratic-republic-of-the-congo-major-cities. Accessed 4 May 2020.
- 26. Fançony C, Gamboa D, Sebastião Y, et al. Various pfcrt and pfmdr1 genotypes of Plasmodium falciparum cocirculate with P. malariae, P. ovale spp., and P. vivax in northern Angola. Antimicrob Agents Chemother 2012; 56:5271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornu M, Combe A, Couprie B, et al. Epidemiological aspects of malaria in 2 villages of the Manyemen forest region (Cameroon, southwest province) [In French]. Med Trop (Mars) 1986; 46:131–40. [PubMed] [Google Scholar]
- 28. Djallé D, Gody JC, Moyen JM, et al. Performance of Paracheck™-Pf, SD Bioline malaria Ag-Pf and SD Bioline malaria Ag-Pf/pan for diagnosis of falciparum malaria in the Central African Republic. BMC Infect Dis 2014; 14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Culleton RL, Mita T, Ndounga M, et al. Failure to detect Plasmodium vivax in West and Central Africa by PCR species typing. Malar J 2008; 7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yman V, Wandell G, Mutemi DD, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl Trop Dis 2019; 13:e0007414.31136585 [Google Scholar]
- 31. Sitali L, Chipeta J, Miller JM, et al. Patterns of mixed Plasmodium species infections among children six years and under in selected malaria hyper-endemic communities of Zambia: population-based survey observations. BMC Infect Dis 2015; 15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruce MC, Macheso A, Kelly-Hope LA, Nkhoma S, McConnachie A, Molyneux ME. Effect of transmission setting and mixed species infections on clinical measures of malaria in Malawi. PLoS One 2008; 3:e2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Omer AH. Species prevalence of malaria in northern and southern Sudan, and control by mass chemoprophylaxis. Am J Trop Med Hyg 1978; 27:858–63. [DOI] [PubMed] [Google Scholar]
- 34. Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasitol Drugs Drug Resist 2013; 3:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Population Commission (NPC), National Malaria Control Programme (NMCP), ICF International. Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: NPC, NMCP, ICF, 2012. [Google Scholar]
- 36. Bray RS. Plasmodium ovale in Liberia. Am J Trop Med Hyg 1957; 6:961–70. [DOI] [PubMed] [Google Scholar]
- 37. Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS One 2014; 9:e87169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 2018; 35:1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clausen PTLC, Aarestrup FM, Lund O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics 2018; 19:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011; 27:2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yager T. The mineral industry of Congo (Kinshasa). In: US Geological Survey, ed. Minerals Yearbook, vol 3, Area Reports–International–Africa and the Middle East. Reston, VA: Government Printing Office, 2013. [Google Scholar]
- 42. Rojo-Marcos G, Rubio-Muñoz JM, Ramírez-Olivencia G, et al. Comparison of imported Plasmodium ovale curtisi and P. ovale wallikeri infections among patients in Spain, 2005–2011. Emerg Infect Dis 2014; 20:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.