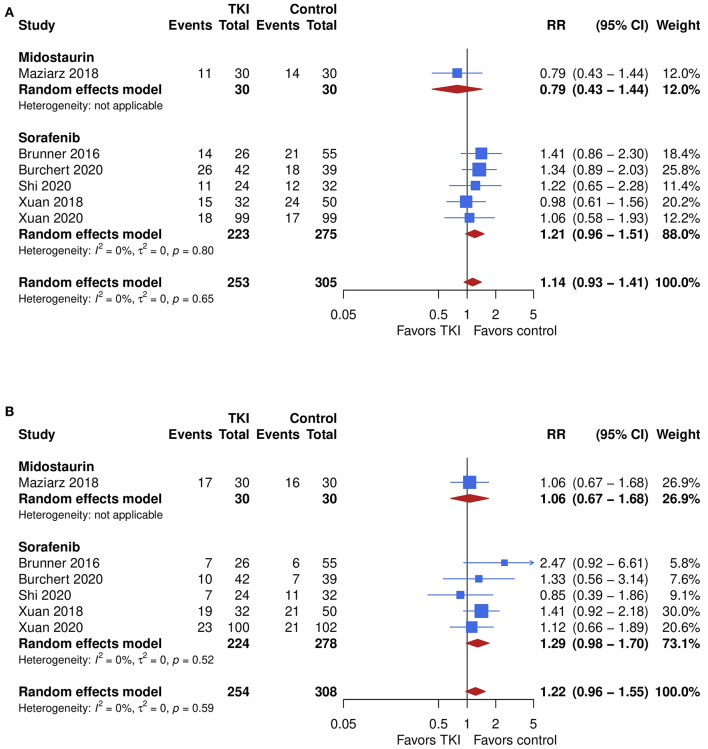
Figure 4.
The impact of TKI therapy on secondary end points of acute and chronic GVHD. Chronic GVHD (A) was assessed in six studies. No significant difference in the incidence was seen, with a trend toward higher incidence after TKI therapy showing an overall pooled RR of 1.14 (95% CI, 0.93–1.41; P = 0.21) with no relevant heterogeneity (I2 = 0%). Subgroup analyses showed no significant difference in outcome between midostaurin and sorafenib (P = 0.19). However, the pooled RR for midostaurin was 0.79 (95% CI, 0.43–1.44) while results for sorafenib suggested higher risk for chronic GVHD showing a RR of 0.43 (95% CI, 0.32–0.57; I2 = 0%), compared with control. Similar results were yielded for acute GVHD (B), which was assessed in six studies. The overall pooled RR was 1.22 (95% CI, 0.96–1.55; P = 0.10) with no relevant heterogeneity (I2 = 0%). No difference was seen between the TKIs (P = 0.48). One study which evaluated midostaurin showed a RR of 1.06 (95% CI, 0.67–1.68), while risk for acute GVHD appeared to be increased after sorafenib therapy showing a RR of 1.29 (95% CI, 0.98–1.70; I2 = 0%), when compared with control.