Abstract
Aims
This study aimed to analyse the clinical presentation and prognosis of patients with Chagas cardiomyopathy and decompensated heart failure (HF), as compared with other aetiologies.
Methods and results
A prospective cohort of patients admitted with decompensated HF. We included 767 patients (63.9% male), with median age of 58 years [interquartile range 48.2–66.7 years]. Main aetiologies were non‐Chagas/non‐ischaemic cardiomyopathies in 389 (50.7%) patients, ischaemic disease in 209 (27.2%), and Chagas disease in 169 (22%). Median left ventricular ejection fraction was 26% (interquartile range 22–35%). Patients with Chagas differed from both patients with non‐Chagas/non‐ischaemic and ischaemic cardiomyopathies for a higher proportion of cardiogenic shock at admission (17.8%, 11.6%, and 11%, respectively, P < 0.001) and had lower blood pressure at admission (systolic blood pressure 90 [80–102.5], 100 [85–110], and 100 [88.2–120] mmHg, P < 0.001) and lower heart rate (heart rate 71 [60–80], 87 [70–102], and 79 [64–96.5] b.p.m., P < 0.001). Further, patients with Chagas had higher serum BNP level (1544 [734–3148], 1061 [465–239], and 927 [369–1455] pg/mL, P < 0.001), higher serum bilirubin (1.4 [0.922.44], 1.2 [0.77–2.19], and 0.84 [0.49–1.45] mg/dL, P < 0.001), larger left ventricular diameter (68 [63–73], 67 [58–74], and 62 [56.8–68.3] mm, respectively, P < 0.001), lower left ventricular ejection fraction (25 [21–30]%, 26 [22–35]%, and 30 [25–38]%, P < 0.001), and a higher proportion of patients with right ventricular function (48.8%, 40.7%, and 25.9%, P < 0.001). Patients with Chagas disease were more likely to receive inotropes than patients with non‐Chagas/non‐ischaemic and ischaemic cardiomyopathies (77.5%, 67.5%, and 62.5%, respectively, P = 0.007) and also to receive intra‐aortic balloon pumping (30.8%, 16.2%, and 10.5%, P < 0.001). Overall, the rates of death or urgent transplant were higher among patients with Chagas than in other aetiologies, a difference that was driven mostly due to increased rate of heart transplant during hospital admission (20.2%, 10.3%, and 8.1%). The prognosis of patients at 180 days after hospital admission was worse for patients with Chagas disease as compared with other aetiologies. In patients with Chagas, age [odds ratio (OR) = 0.934, confidence interval (CI)95% 0.901–0.982, P = 0.005], right ventricular dysfunction by echocardiography (OR = 2.68, CI95% 1.055–6.81, P = 0.016), and urea (OR = 1.009, CI95% 1.001–1.018, P = 0.038) were significantly associated with prognosis.
Conclusions
Patients with Chagas cardiomyopathy and decompensated HF have a distinct clinical presentation and worse prognosis compared with other aetiologies.
Keywords: Chagas disease, Heart failure, Decompensated heart failure, Prognosis, Risk
Introduction
Chagas disease is a clinical condition of growing epidemiological importance. In Latin America alone, it is estimated that 6 million people have been infected with Trypanosoma cruzi 1 and an unknown number of individuals are silent carriers; more recently, migration has further contributed to the dissemination of the disease, 2 and currently 400 000 infected individuals are thought to live in non‐endemic areas. 3 Importantly, it is estimated that up to 20–30% of the infected individuals may eventually develop cardiac disease, and Chagas cardiomyopathy is reported to be the cause of heart failure (HF) in up to 25% of the HF cases in referral centres. 4
Chronic Chagas cardiomyopathy is thought to have a distinct presentation when compared with other HF aetiologies. Patients with Chagas are younger and have less co‐morbidities but have more frequently bradycardia, conduction abnormalities, arterial hypotension, right ventricular dysfunction, and thromboembolic events, among others. 4 , 5 Importantly, patients with chronic HF due to Chagas cardiomyopathy have worse prognosis as compared with other aetiologies; the all‐cause mortality rates at 1, 5, and 10 years of follow‐up are thought to be approximately 12%, 35%, and 60%, respectively, 6 and the 1 year all‐cause mortality rate may be as high as 90% in patients with more severe forms of the disease. 7 , 8 However, it has been suggested that the risk factors found in patients with chronic HF and Chagas disease may differ from traditional HF risk factors, 9 with significant impact on mode of death 10 and response to therapeutic interventions, 11 , 12 further emphasizing the importance of the distinctive clinical features of this disease.
These clinical differences are the result of pathophysiologic, functional, and structural derangements found in patients after trypanosomal infection and may include a direct effect of the parasite on cardiac and neural cells, increased inflammatory and neurohumoral activation, 13 sympathetic denervation, and thrombogenic status, 14 among others. 5 Interestingly, many—if not all—of these factors are exacerbated during episodes of acute decompensated HF and, therefore, have the potential of further influencing the clinical presentation and prognosis of patients. Despite recent suggestions that the clinical presentation of patients with Chagas heart disease and decompensated HF may in fact differ from other aetiologies, 15 the clinical significance of these findings, especially in terms of the impact on risk stratification, therapy response, and prognosis, has not been sufficiently analysed.
We hypothesized that patients with Chagas cardiomyopathy may have a distinct presentation during episodes of decompensated HF influencing the prognosis, risk stratification, and response to therapy.
Methods
Objectives
The primary aim of our study was to evaluate the course of patients with Chagas disease during episodes of decompensated HF, as compared with other aetiologies. Further, we sought to investigate the presence markers associated with in‐hospital prognosis; exploratory analysis was also performed for the prognosis after hospital discharge.
Study design
We analysed a prospective cohort of patients admitted to the Heart Institute (InCor) of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC‐FMUSP; online registry 13713) with a diagnosis of acute decompensated HF. Our centre is a 535‐bed tertiary academic hospital dedicated to cardiology and a referral institution for all regions of the country. The selection of patients was performed by means of active monitoring of the medical and administrative databases of our institution. The first inclusion occurred in August 2013, and the last was in January 2018. The study was approved by the Institutional Ethics Committee for Research Project Analysis, and patients were followed up for 180 days after hospital discharge.
Inclusion and exclusion criteria
We included patients over 18 years of age admitted with a diagnosis of acute decompensated HF and ejection fraction less than 50% as measured by echocardiography. We excluded patients hospitalized for less than 24 h and patients with cardiogenic shock or decompensated HF during the post‐operative period after heart surgery. For the purpose of the present analysis, HF aetiology was categorized into three groups: Chagas cardiomyopathy, ischaemic cardiomyopathy, and non‐Chagas/non‐ischaemic cardiomyopathy.
Analysed variables
Data were obtained from medical records, including demographic information, epidemiological data, pathological history, reason for hospitalization, presence and duration of HF‐related symptoms, aetiologic diagnosis of HF or cardiomyopathy, physical examination, and electrocardiographic and echocardiographic data. The estimation of right ventricular function was based on subjective analysis; for the purpose of present analysis, the presence of right ventricular dysfunction was defined as echocardiographic dysfunction estimated as moderate or severe, and the absence of right ventricular dysfunction was defined as echocardiographic dysfunction estimated as mild or absent. The occurrence of death and heart transplantation since hospital admission up to 180 days after hospital discharge was registered.
Statistical analysis
Categorical variables are described as absolute value and percentage; continuous variables are described as median ± interquartile range [IQR] 25–75%. For non‐normal distribution of variables, the non‐parametric Mann–Whitney U test was used. Comparison of proportions between groups was performed with the χ² test. In order to correct for multiple comparisons, the step‐down Bonferroni–Holm procedure was applied to each set of measurements. Both adjusted and unadjusted P‐values are reported. Multivariable analysis was performed with logistic regression; all variables with clinical significance were entered in the model. Survival was estimated by using the Kaplan–Meier method, and differences in survival between groups were assessed with the log‐rank test. P‐values less than 0.05 were considered significant. In order to analyse the prognosis of patients in the follow‐up, a Cox regression model was performed for the occurrence of the combined endpoint of death, heart transplant, and hospital readmission; all variables with clinical significance were entered in the model. Statistical analysis was performed using SPSS for Windows Version 11.0.
Results
Baseline characteristics
We included 767 patients admitted with decompensated HF from August 2013 through January 2018 (Table 1 ); 80 (10.4%) had the diagnosis of de novo HF. Patients were predominantly male (63.9%), and the median age was 58 years (IQR 48.2–66.7 years). Main aetiologies were non‐Chagas/non‐ischaemic cardiomyopathy in 389 (52.4%) patients, ischaemic heart disease in 209 (27.2%), and Chagas disease in 169 (22%). Median left ventricular (LV) ejection fraction was 26% (IQR 22–35%). Inotropes were used in 523 (68.2%) and intra‐aortic balloon pump in 137 (17.9%) patients; 224 (29.2%) died during hospital admission, and 91 (11.9%) were transplanted.
Table 1.
Clinical characteristics of patients
| Clinical characteristics | Total |
|---|---|
| Median [IQR]/N (%) | |
| Number of patients | 767 |
| Gender | |
| Female | 277 (36.1) |
| Male | 490 (63.9) |
| Age (years) | 58 [48.2–66.7] |
| Co‐morbidities | |
| Arterial hypertension | 402 (52.4) |
| Diabetes mellitus | 239 (31.2) |
| Atrial fibrillation | 279 (36.4) |
| HF aetiology | |
| Non‐ischaemic/non‐Chagas | 389(50.7) |
| Ischaemic heart disease | 209 (27.2) |
| Chagas heart disease | 169 (22) |
| Medications | |
| Beta‐blocker | 625 (81.5) |
| ACEi/ARB | 503 (65.6) |
| Hydralazine/nitrate | 559 (72.9) |
| Spironolactone | 440 (57.4) |
| Diuretics | 601 (78.4) |
| Digoxin | 182 (23.7) |
| Warfarin | 201 (26.2) |
| Acetylsalicylic acid | 251 (32.7) |
| Cardiac devices | |
| ICD | 59 (7.7) |
| CRT‐D | 41 (5.3) |
| Admission diagnosis | |
| Progressive HF | 461 (60.1) |
| Cardiogenic shock | 98 (12.8) |
| Arrhythmia/syncope | 83 (10.8) |
| ACS | 31 (4.0) |
| Others | 94 (12.3) |
| Physical examination | |
| Congestion | 628 (81.8) |
| Hypoperfusion | 277 (36.1) |
| SBP (mmHg) | 100 [84–111.5] |
| Heart rate (b.p.m.) | 80 [68–98] |
| Laboratory findings (serum) | |
| Creatinine (mg/dL) | 1.64 [1.21–2.38] |
| Urea (mg/dL) | 75 [49–113] |
| Sodium (mEq/L) | 137 [133–140] |
| Potassium (mEq/L) | 4.4 [4.0–4.9] |
| BNP (pg/dL) | 1069 [474–2028] |
| Echocardiographic findings | |
| LV ejection fraction (%) | 26 [22–35] |
| RV dysfunction | 289 (37.7) |
| Prognosis | |
| Inotropes | 523 (68.2) |
| IABC | 137 (17.9) |
| Dialysis | 113 (14.7) |
| In‐hospital mortality | 224 (29.2) |
| Urgent heart transplant | 91 (11.9) |
ACEi, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; CRT‐D, cardiac resynchronization therapy in association with a defibrillator; HF, heart failure; IABC, intra‐aortic balloon; ICD, intracardiac defibrillator; IQR, interquartile range; LV, left ventricle; RV, right ventricle; SBP, systolic blood pressure.
Comparison of clinical and complementary variables
When clinical characteristics were analysed according to the aetiology (Table 2 ), we found that patients with Chagas disease, as well as patients with non‐Chagas/non‐ischaemic cardiomyopathy, were younger as compared with patients with ischaemic heart disease (ages 57 [IQR 46.2–64.7], 55 [43–65], and 64 [57–71] years old, respectively, P < 0.001) and had a higher proportion of female patients (42.6%, 39.1%, and 25.4%, respectively, P < 0.001). Importantly, patients with Chagas differed from both patients with non‐Chagas/non‐ischaemic cardiomyopathy and patients with ischaemic cardiomyopathy for a higher proportion of individuals with syncope at admission (20.2%, 12.6%, and 11.5%, respectively, P = 0.028), lower proportion of diabetes mellitus (13%, 26%, and 55.8%, respectively, P < 0.001) and arterial hypertension (30.2%, 50.4%, and 74.2%, respectively, P < 0.001), and higher proportion of patients with admission diagnosis of cardiogenic shock (17.8%, 11.6%, and 11%, respectively, P < 0.001) or arrhythmias and syncope (18.9%, 6.7%, and 12%, respectively, P < 0.001); additionally, patients with Chagas were more likely to have a lower blood pressure at admission (systolic blood pressure 90 [80–102.5], 100 [85–110], and 100 [88.2–120] mmHg, respectively, P < 0.001) and a lower heart rate (heart rate 71 [60–80], 87 [70–102], and 79 [64–96.5] b.p.m., respectively, P < 0.001). Except for angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, all other medications that patients received prior to hospital admission differed among groups.
Table 2.
Clinical characteristics of patients according to aetiology
| Clinical characteristics | Chagas | Non‐Chagas/non‐ischaemic | Ischaemic | P‐value | Adjusted P‐value |
|---|---|---|---|---|---|
| Median [IQR]/N (%) | Median [IQR]/N (%) | Median [IQR]/N (%) | |||
| Number of patients | 169 | 389 | 209 | ||
| Gender | |||||
| Female | 72 (42.6) | 152 (39.1) | 53 (25.4)*** | <0.001 | 0.003 |
| Male | 97 (57.4) | 237(60.9) | 156 (74.6) | ||
| Age (years) | 57 [46.2–64.7] | 55 [43–65] | 64 [57–71]*** | <0.001 | <0.001 |
| Duration of symptoms | 8 [3–31] | 14 [4–31] ++ | 7 [2–28.9] | 0.015 | 0.06 |
| Symptoms at presentation | 0.028 | 0.084 | |||
| Chest pain | 41 (24.6) | 105 (27.2) | 71 (34.1) | 0.09 | 0.09 |
| Orthopnoea | 91 (53.8) | 214 (55) | 88 (42.1) | 0.008 | 0.04 |
| PND | 78 (46.2) | 198 (51) | 77 (36.8)* | 0.004 | 0.024 |
| Syncope | 34 (20.2) | 49 (12.6) ++ | 24 (11.5)* | 0.028 | 0.08 |
| Co‐morbidities | |||||
| Arterial hypertension | 51 (30.2) | 196 (50.4) +++ | 155 (74.2)*** | <0.001 | <0.001 |
| Diabetes mellitus | 22 (13) | 101 (26) + | 116 (55.8)*** | <0.001 | <0.001 |
| Dyslipidaemia | 28 (16.9) | 76 (19.8) | 117 (57.1)*** | <0.001 | <0.001 |
| Atrial fibrillation | 69 (41.6) | 146 (38.2) | 64 (30.8)* | 0.074 | 0.074 |
| Previous VT/VF | 30 (17.8) | 29 (7.5) ++ | 39 (18.8) | <0.001 | <0.001 |
| Admission diagnosis | +++ | *** | |||
| Progressive HF | 89 (52.7) | 267 (68.6) | 105 (50.2) | ||
| Cardiogenic shock | 30 (17.8) | 45 (11.6) | 23 (11) | ||
| Arrhythmia/syncope | 32 (18.9) | 26 (6.7) | 25 (12) | <0.001 | <0.001 |
| ACS | 2 (1.2) | 8 (2.1) | 21 (10) | ||
| Others | 16 (9.5) | 43 (11.1) | 35 (16.7) | ||
| Physical exam | |||||
| Congestion | 135 (79.9) | 333 (85.6) | 159 (76.1) | 0.012 | 0.0024 |
| Hypoperfusion | 78 (46.2) | 140 (36.5) + | 59 (28.2)*** | 0.002 | 0.006 |
| SBP (mmHg) | 90 [80–102.5] | 100 [85–110] +++ | 100 [88.2–120]*** | <0.001 | <0.001 |
| Heart rate (b.p.m.) | 71 [60–86] | 85 [70–102] +++ | 79 [64–96.5]** | <0.001 | <0.001 |
| Mitral regurgitation | 68 (40.2) | 121 (31.2) | 40 (19.2)*** | <0.001 | 0.0011 |
| Tric. regurgitation | 16 (9.5) | 30 (7.7) | 15 (7.2) | 0.79 | 0.79 |
| Previous medications | |||||
| ACEi/ARB | 121 (71.6) | 254 (65.3) | 128 (61.2)* | 0.107 | 0.146 |
| Beta‐blocker | 150 (88.8) | 317 (81.5) + | 158 (75.6)* | 0.005 | 0.02 |
| Hydralazine/nitrate | 40 (23.7) | 99 (25.4) | 69 (33)* | 0.073 | 0.146 |
| Spironolactone | 107 (63.3) | 228 (58.3) | 105 (50.2)* | 0.03 | 0.09 |
| Diuretics | 139 (82.2) | 315 (81) | 147 (70.3)** | 0.004 | 0.02 |
| Digoxin | 42 (24.9) | 113 (29) | 27 (12.9)** | <0.001 | <0.001 |
| Warfarin | 54 (32) | 110 (28.3) | 37 (17.7)** | 0.003 | 0.018 |
| Acetylsalicylic acid | 33 (19.5) | 94 (24.2) | 124 (59.3)*** | <0.001 | <0.001 |
| Amiodarone | 58 (34.3) | 57 (14.7) +++ | 30 (14.4)*** | <0.001 | <0.001 |
ACEi, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; HF, heart failure; IQR, interquartile range; PND, paroxysmal nocturnal dyspnoea; SBP, systolic blood pressure; VF, ventricular fibrillation; VT, ventricular tachycardia.
Valvular murmurs estimate as moderate/severe.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. ischaemic.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. non‐Chagas/non‐ischaemic.
Regarding laboratory findings, the presentation of patients with Chagas cardiomyopathy differed from other aetiologies and was remarkable for higher serum BNP level (1544 [734–3148], 1061 [465–239], and 927[369–1455] pg/mL, respectively, P < 0.001) and higher serum bilirubin (1.4 [0.92–2.44], 1.2 [0.77–2.19], and 0.84 [0.49–1.45] mg/dL, respectively, P < 0.001), among others (Table 3 ).
Table 3.
Laboratory characteristics of patients according to aetiology
| Chagas | Non‐Chagas/non‐ischaemic | Ischaemic | P‐value | Adjusted P‐value | |
|---|---|---|---|---|---|
| Median [IQR]/N (%) | Median [IQR]/N (%) | Median [IQR]/N (%) | |||
| Number of patients | 169 | 389 | 209 | ||
| Creatinine (mg/dL) | 1.58 [1.23–2.22] | 1.58 [1.16–2.42] | 1.78 [1.3–2.41] | 0.168 | 0.84 |
| Urea (mg/dL) | 71 [49–100] | 74 [47–116] | 80 [51–125] | 0.327 | 0.99 |
| Sodium (mEq/L) | 137 [133–140] | 136 [133–140] | 138 [135–140] | 0.02 | 0.22 |
| eGFR | 44.7 [28.1–58.9] | 45.4 [26.1–64.5] | 36.8 [25.5–52.7]* | 0.03 | 0.27 |
| Potassium (mEq/L) | 4.4 [4.0–4.8] | 4.4 [3.9–5.0] | 4 [4–4.9] | 0.99 | 0.99 |
| BNP (pg/dL) | 1545 [734–3148] | 1061 [465–239] +++ | 927 [369–1455]*** | <0.001 | <0.001 |
| Leucocytes | 7210 [5680–8990] | 7825 [527–10 269] + | 8715 [6285–11 557]*** | <0.001 | 0.015 |
| Haemoglobin | 13 [12–14.5] | 13 [11–15] | 13 [12–14] | 0.69 | 0.99 |
| Cholesterol (mg/dL) | 127 [110–169] | 135 [111–169] + | 133 [106.7–165.2] | 0.675 | 0.99 |
| Triglycerides (mg/dL) | 72 [55–93] | 83 [61–110] + | 93 [65–129]** | 0.001 | 0.02 |
| Glycaemia (mg/dL) | 103 [85.5–120] | 102 [88–125] | 122 [102–160]*** | <0.001 | <0.001 |
| Bilirubin (mg/dL) | 1.4 [0.92–2.44] | 1.2 [0.77–2.19] | 0.84 [0.49–1.45]*** | <0.001 | 0.048 |
| GGT (mg/dL) | 217 [119–358] | 151 [96–274.8] ++ | 145 [62–246]*** | <0.001 | 0.026 |
| AST (mg/dL) | 37 [22–80] | 33 [22–80] | 31 [21–62] | 0.130 | 0.84 |
| ALT (mg/dL) | 39 [24–74.3] | 34 [25–61.5] | 31 [22–54]* | 0.035 | 0.28 |
| Albumin (mg/dL) | 3.1 [2.77–3.5] | 3 [2.6–3.4] | 3 [2.3–3.45] | 0.025 | 0.25 |
| HbA1c (%) | 6.1 [5.7–6.6] | 6.1 [5.7–6.7] | 6.3 [5.8–7.85] | 0.12 | 0.84 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimate glomerular filtration rate; GGT, gamma‐glutamyl transferase; HbA1c, glycated haemoglobin; IQR, interquartile range.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. ischaemic.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. non‐Chagas/non‐ischaemic.
Regarding echocardiographic parameters (Table 4 ), patients with Chagas disease showed increased remodelling parameters, as indicated by larger LV diameter (68 [63–73], 67 [58–74], and 62 [56.8–68.3] mm, respectively, P < 0.001); additionally, ventricular function was more pronouncedly reduced among patients with Chagas, as indicated by lower LV ejection fraction (25 [21–30]%, 26 [22–35]%, and 30 [25–38]%, P < 0.001) and a higher proportion of patients with right ventricular function estimated as moderately or severely reduced (48.8%, 40.7%, and 25.9%, P < 0.001).
Table 4.
Echocardiographic findings according to aetiology
| Clinical characteristics | Chagas | Non‐Chagas/non‐ischaemic | Ischaemic | P‐value | Adjusted P‐value |
|---|---|---|---|---|---|
| Median [IQR]/N (%) | Median [IQR]/N (%) | Median [IQR]/N (%) | |||
| Number of patients | 169 | 389 | 209 | ||
| Septum (mm) | 8 [8–9] | 9 [8–11] +++ | 9 [8–10]*** | <0.001 | 0.06 |
| LV posterior wall (mm) | 8 [7–9] | 9 [8–10] +++ | 9 [8–10]*** | <0.001 | 0.02 |
| LVDD (mm) | 68 [63–73] | 67 [58–74] | 62 [56.8–68.3]*** | <0.001 | 0.0015 |
| LVEF (%) | 25 [21–30] | 26 [22–35] + | 30 [25–38]*** | <0.001 | 0.0015 |
| LA (mm) | 49 [45–54] | 50 [45–55] | 49 [45–53] | 0.27 | 0.54 |
| PASP (mmHg) | 45 [35–52] | 48 [39–57] ++ | 48 [37.8–60]* | 0.011 | 0.045 |
| RV dysfunction (%) a | 81 (48.8) | 156 (40.7) + | 52 (25.9)*** | <0.001 | <0.001 |
| Mitral regurgitation (%) a | 114 (67.5) | 211 (54.2) + | 106 (50.7)** | 0.009 | 0.045 |
| Tricuspid regurgitation (%) a | 103 (60.9) | 179 (46) ++ | 73 (34.9)*** | <0.001 | <0.001 |
| Intracavitary thrombus (%) | 13 (8) | 27 (7.3) | 13 (6.5) | 0.85 | 0.85 |
DD, diastolic diameter; EF, ejection fraction; IQR, interquartile range; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; RV, right ventricle.
Estimated as moderate or severe.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. ischaemic.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. non‐Chagas/non‐ischaemic.
Invasive haemodynamic evaluation was available in 187 (24.4%) and showed (Table 5 ) that patients with Chagas disease, when compared with patients with dilated and ischaemic aetiologies, had lower heart rate (88 [70–99], 104 [93–110], and 95.5 [90.5–106.8] b.p.m., P < 0.001) and lower systolic pulmonary pressure (47 [35–59], 50 [42–61], and 60 [41.5–70.5] mmHg, P = 0.0018).
Table 5.
Haemodynamic findings according to aetiology
| Clinical characteristics | Chagas | Non‐Chagas/non‐ischaemic | Ischaemic | P‐value | Adjusted P‐value |
|---|---|---|---|---|---|
| Median [IQR]/N (%) | Median [IQR]/N (%) | Median [IQR]/N (%) | |||
| Number of patients | 52 | 90 | 45 | ||
| Heart rate (b.p.m.) | 88 [70–99] | 104 [93–110] +++ | 95.5 [90.5–106.8]* | <0.001 | <0.001 |
| Systolic systemic AP (mmHg) | 94 [8–9] | 96 [87–105.5] | 100 [90–111.7] | 0.26 | 0.99 |
| Mean systemic AP (mmHg) | 70.5 [65.3–78.8] | 72 [67–80] | 67.5 [61.5–83] | 0.30 | 0.99 |
| Systolic pulmonary AP (mmHg) | 47 [35–59] | 50 [42–61] | 60 [41.5–70.5]* | 0.018 | 0.126 |
| Mean pulmonary AP (mmHg) | 35 [25–42.5] | 36 [29.8–42] | 40 [30–48]* | 0.056 | 0.34 |
| Wedge pressure (mmHg) | 24 [17–28.8] | 25 [18.5–30] | 22 [16–32] | 0.408 | 0.99 |
| Right atrium pressure (mmHg) | 14 [10–19.2] | 13 [9–19] | 12.5 [8–16.8] | 0.464 | 0.99 |
| Cardiac output (L/min) | 3.6 [3–4.3] | 4 [2.9–5.1] | 4.25 [3.3–5.3]* | 0.078 | 0.39 |
AP, arterial pressure; IQR, interquartile range.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. ischaemic.
P < 0.05.
P < 0.01.
P < 0.001 for Chagas vs. non‐Chagas/non‐ischaemic.
In‐hospital interventions and prognosis
Patients with Chagas disease were more likely to receive inotropes than patients with dilated and ischaemic cardiomyopathies (77.5%, 67.5%, and 62.5%, respectively, P = 0.007) and also to receive intra‐aortic balloon pumping (30.8%, 16.2%, and 10.5%, respectively, P < 0.001). The rates of death or urgent transplant were higher among patients with Chagas than in other aetiologies (Figures 1 , 2 , 3 , 4 , 5 ); the higher mortality was mostly linked to the higher heart transplant rate during hospital admission: 52 (30.8%) patients with Chagas died and 34 (20.2%) were transplanted, 104 (26.7%) patients with dilated cardiomyopathies died and 40 (10.3%) were transplanted, and 68 (32.5%) patients with ischaemic cardiomyopathy died and 17 (8.1%) were transplanted. The prognosis of patients at 180 days after hospital admission was also worse for patients with Chagas disease as compared with other aetiologies (Figures 2 , 3 , 4 , 5 ).
Figure 1.
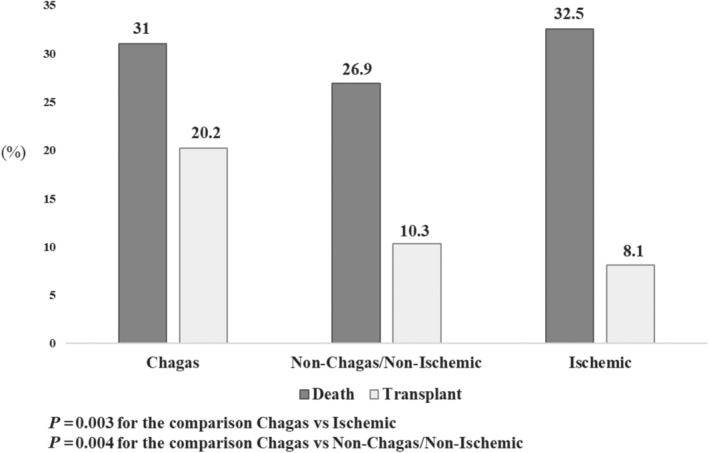
In‐hospital prognosis according to aetiology.
Figure 2.
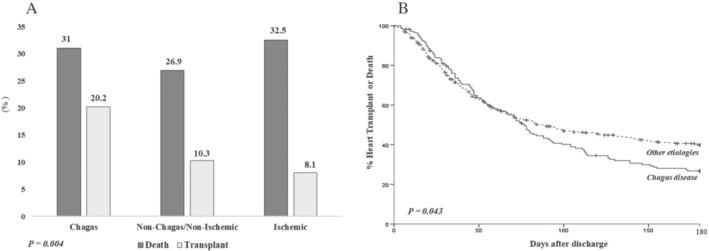
Central illustration. Prognosis of patients with Chagas cardiomyopathy compared with other aetiologies after an episode of acute decompensated heart failure. (A) In‐hospital prognosis according to aetiology. (B) Kaplan–Meier curve depicting the survival of patients according to aetiology at 180 days of follow‐up.
Figure 3.
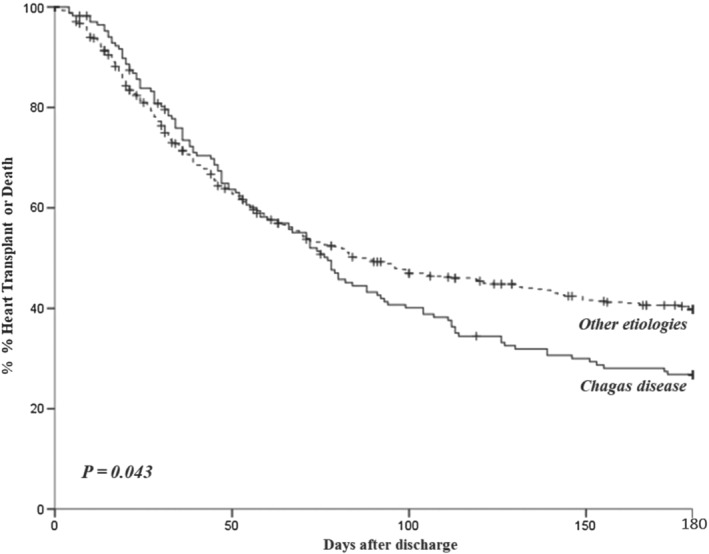
Kaplan–Maier curve depicting the survival of patients according to aetiology at 180 days of follow‐up.
Figure 4.
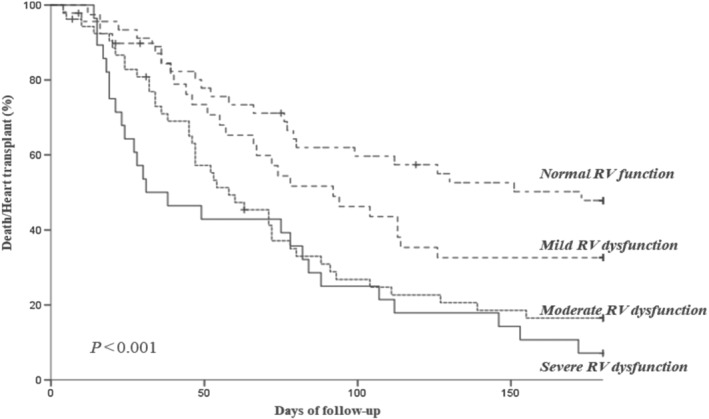
Rate of death and urgent heart transplant according to the presence of right ventricular (RV) dysfunction in echocardiography in patients with Chagas disease.
Figure 5.
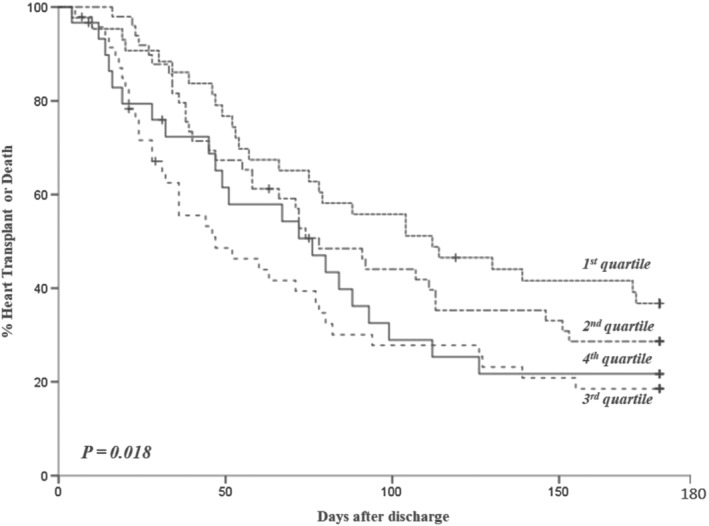
Rate of death and urgent heart transplant in patients with Chagas disease according to the urea quartiles at admission.
Risk stratification
A logistic regression analysis for adverse in‐hospital composite outcome (death plus heart transplantation) was performed (Table 6 ). All variables with clinical significance were entered in the model, including age, systolic arterial pressure, urea serum level, BNP level at hospital admission, pulmonary systolic pressure as measured by echocardiography, and presence of right ventricular dysfunction and LV ejection fraction by echocardiography. We found that in patients with Chagas heart disease, age [odds ratio (OR) = 0.941, confidence interval (CI)95% 0.901–0.982, P = 0.005], presence of right ventricular dysfunction estimated as moderate or severe at echocardiography (OR = 2.68, CI95% 1.055–6.81, P = 0.025), and serum urea (OR = 1.009, CI95% 1.001–1.018, P = 0.038) were significantly associated with prognosis. In patients with other aetiologies, the variables associated with prognosis were arterial systolic pressure (OR = 0.975, CI95% 0.964–0.987, P < 0.001), LV ejection fraction (OR = 0.972, CI95% 0.974–0.999, P = 0.041), and BNP level (OR = 1.545, CI95% 1.177–2.029, P = 0.002). In order to investigate the significance of these variables only for mortality, we performed a logistic regression analysis for the occurrence of in‐hospital death. In patients with Chagas, the serum urea was associated with increased mortality (OR = 1.011, CI95% 1.001–1.021, P = 0.026), and in patients with other aetiologies, the arterial systolic pressure (OR = 0.974, CI95% 0.962–0.987, P < 0.001), age (OR = 1.023, CI95% 1.002–1.043, P = 0.03), serum BNP (OR = 1.768, CI95% 1.293–2.417, P < 0.001), and the pulmonary systolic pressure (OR = 1.022, CI95% 1.002–1.041, P = 0.028) were associated with mortality.
Table 6.
Logistic regression analysis for adverse in‐hospital composite outcome (death plus heart transplantation) according to aetiology
| Variable | Chagas cardiomyopathy | Other aetiologies | ||||
|---|---|---|---|---|---|---|
| OR | IC95% | P‐value | OR | IC95% | P‐value | |
| Age | 0.941 | 0.901–0.982 | 0.005 | 1.007 | 0.989–1.025 | 0.456 |
| Systolic arterial pressure | 0.988 | 0.963–1.014 | 0.361 | 0.975 | 0.964–0.987 | <0.001 |
| Urea | 1.009 | 1.001–1.018 | 0.038 | 1.002 | 0.998–1.007 | 0.295 |
| BNP | 1.117 | 0.672–1.858 | 0.669 | 1.545 | 1.177–2.029 | 0.002 |
| Pulmonary systolic pressure | 0.968 | 0.939–1.011 | 0.164 | 1.018 | 1.000–1.035 | 0.045 |
| Right ventricular dysfunction | 2.68 | 1.055–6.810 | 0.016 | 1.316 | 0.783–2.11 | 0.3 |
| Left ventricular ejection fraction | 1.009 | 0.957–1.064 | 0.735 | 0.972 | 0.974–0.999 | 0.041 |
CI, confidence interval; OR, odds ratio.
The odds ratios in the logistic regression correspond to one‐unit increase in the covariate.
We further analysed these variables in a Cox regression model for events in the follow‐up (death, heart transplant, and hospital readmission); we found that age (HR 0.98 [0.962–0.999], P = 0.042), right ventricular dysfunction (HR 1.672 [1.034–2.705], P = 0.036), and urea (HR 1.004 [1.001–1.008], P = 0.012) were independently associated with prognosis in the first 180 days of follow‐up (Appendix A and Figures 4 and 5 ).
Discussion
The analysis of this cohort of 767 patients showed that patients with Chagas cardiomyopathy have a distinctive clinical during episodes of HF decompensation, as indicated by younger age, higher proportion of female gender, lower frequency of co‐morbidities such as diabetes mellitus and arterial hypertension, higher proportion of patients with cardiogenic shock and syncope at admission, and lower blood pressure and heart rate. Further, patients with Chagas had more frequently markers of advanced cardiac disease at presentation, as suggested by a higher BNP level, increased LV dimension, and worse biventricular function in the echocardiogram. Most importantly, patients with Chagas aetiology had a higher death rate and heart transplant during hospital stay as well as at 180 days after hospital discharge. Prognostic markers were also distinct in patients with Chagas and were mostly associated with right ventricular function. The major strength of the present study was the inclusion of a significant number of patients with Chagas disease during episodes of decompensated HF, a clinical scenario scarcely explored in previous studies; it also presents a thorough examination of the patients, including clinical, haemodynamic, echocardiographic, and prognostic information.
Some particular characteristics of the present cohort should be acknowledged. Firstly, patients tended to be young (58 [48.2–66.7] years) and with a higher proportion of male patients (63.9%); the in‐hospital mortality was high (29.2%), as well as the frequency of inotropic therapy and heart transplantation, which reflect the fact that our institution is a tertiary referral centre that cares for high‐risk patients. These findings contrast with reports from other studies. Data from the ADHERE registry that included patients from the USA showed a mean age of 72 years, a higher prevalence of female patients, ischaemic heart disease as the main aetiology, and in‐hospital mortality of 4%. 16 Similarly, the reported mean age from the European registry was 70 ± 13 years with a majority of male patients; the total in‐hospital mortality rate was 3.8%. 17 Data from the Brazilian Registry of patients with decompensated HF showed mean age of 64 years, a predominance of male patients, and in‐hospital mortality of 12.6%. 18 These differences may be mostly due to the inclusion of a high proportion of patients with Chagas. 19 There are few other studies on the patients with Chagas heart disease in the scenario of acute decompensation; data derived from studies with patients with chronic HF indicate that patients with Chagas may have a worse prognosis as compared with other aetiologies. 13 , 20 In the setting of decompensated HF, a recent study compared the prognosis of patients with Chagas cardiomyopathy with that of patients with other aetiologies; no difference was found regarding in‐hospital mortality, but patients with Chagas had a higher rate of hospital readmission. 21 Additionally, it should be noticed that our centre is a tertiary hospital dedicated to cardiology that care for patients with advanced HF, with a higher expected mortality compared with that in community hospitals. In this sense, a majority of our patients received inotropes during their hospital stay (68.2%), and a significant proportion received either an intra‐aortic balloon (17.9%) or dialysis (14.7%).
One important finding was the reported higher rate of the outcomes of death or heart transplant during hospital stay, as well as at 180 days of follow‐up after hospital discharge among patients with Chagas disease. This difference was driven mostly by a higher rate of heart transplantations as compared with other aetiologies. Other studies have explored the association between HF aetiology and outcomes, and most previous comparisons from the USA and Europe have indicated a lower survival among patients with ischaemic heart disease. 22 , 23 , 24 In this respect, studies from areas where Chagas disease is an endemic condition have reported a worse prognosis among patients with Chagas, including excessive mortality and higher rates of readmission. 25 Our findings confirm that patients with Chagas have a worse prognosis during episodes of decompensated HF; different mechanisms have been proposed for this worse prognostic found in patients with Chagas, and most information comes from patients with chronic Chagas cardiomyopathy. 13 Possible mechanisms reported may include an increased rate of right ventricular dysfunction, ventricular arrhythmias, and conduction abnormalities as well as higher rate of thromboembolic events. 11 , 26
In the present analysis, we sought to identify clinical markers associated with clinical outcomes during hospital admission among patients with Chagas aetiology, and we found that a younger age, presence of significant right ventricular dysfunction by echocardiography, and renal function were independently associated with the occurrence of death or heart transplant. Previous studies have identified clinical markers in populations where the ischaemic aetiology is more frequent, and reported variables associated with worse prognosis in the setting of acute HF include arterial blood pressure, renal function, and LV function, among others. 27 , 28 The importance of the right ventricular function in patients with chronic Chagas disease had been previously demonstrated; pathology findings suggest that the right ventricle is involved early during the course of the disease 29 ; more recently, studies using magnetic resonance imaging 30 and speckle‐tracking echocardiography 31 have further confirmed these findings. However, the clinical and prognostic relevance of right ventricular involvement in the setting of acute decompensated HF had not been previously reported. Similarly, the clinical relevance of renal dysfunction among patients with Chagas had been reported in patient with chronic HF, 32 but its significance in the decompensated patient had not yet been studied. The presence of increasing age as a protective prognostic marker probably reflects the increased chance of younger patients to be admitted to a heart transplant protocol, reaching, thus, one of the studied outcomes. Finally, it is possible to consider that the socio‐economic conditions differ in patients with Chagas disease as compared with other aetiologies and may modulate the clinical presentation of the disease with access to therapy and better prognosis. 33
There are limitations in the present study that should be acknowledged; we included a limited number of patients compared with other HF patient cohorts, and despite the continuous surveillance of patients admitted to our institution, we cannot exclude the possibility of loss of patients at inclusion; therefore, the possibility of selection bias cannot be excluded. Additionally, as clinical data were obtained from medical records, heterogeneity regarding information from anamnesis and physical examination cannot be excluded. Information about the treatment that patients were receiving on the day they had the haemodynamic measurements performed could not be retrieved; therefore, the results of the haemodynamic study may have been influenced by therapeutic interventions, such as inotropic therapy and presence of intra‐aortic balloon. Finally, some particular characteristics of our centre (a tertiary academic institution dedicated to cardiology) and of our population (younger age and the high‐risk patient profile) may hinder the applicability of our results to other populations.
Conclusions
Our results indicate that during episodes of decompensated HF, patients with Chagas cardiomyopathy have a distinct clinical presentation and worse prognosis as compared with other aetiologies; reduced right ventricular function and impaired renal function are important prognostic markers. These results should be taken into consideration in the clinical and therapeutic approach to patients with Chagas hospitalized for HF.
Conflict of interest
The authors have no conflicts of interest to disclose.
Funding
There were no external sources of funding for this manuscript.
Appendix A. Cox regression model for events in the follow‐up in patients with Chagas disease
| Variable | HR | CI95% | P‐value |
|---|---|---|---|
| Age | 0.980 | 0.962–0.999 | 0.042 |
| Right ventricular dysfunction | 1.672 | 1.034–2.705 | 0.036 |
| Urea | 1.004 | 1.001–1.008 | 0.012 |
| Pulmonary systolic pressure | 1.008 | 0.989–1.028 | 0.398 |
| BNP | 1.073 | 0.81–1.421 | 0.623 |
| Systolic arterial pressure | 0.996 | 0.984–1.007 | 0.443 |
| Left ventricular ejection fraction | 0.992 | 0.962–1.022 | 0.589 |
CI, confidence interval; HR, hazard ratio.
The hazard ratios in the logistic regression correspond to one‐unit increase in the covariat
Issa, V. S. , Ayub‐Ferreira, S. M. , Schroyens, M. , Chizzola, P. R. , Soares, P. R. , Lage, S. H. G. , and Bocchi, E. A. (2021) The course of patients with Chagas heart disease during episodes of decompensated heart failure. ESC Heart Failure, 8: 1460–1471. 10.1002/ehf2.13232.
References
- 1. World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 2015; 90: 33–43. PMID: 25671846. [PubMed] [Google Scholar]
- 2. Antinori S, Galimberti L, Grande R, Bianco R, Oreni L, Traversi L, Ricaboni D, Bestetti G, Lai A, Mileto D, Gismondo MR, Petullà M, Garelli S, de Maio G, Cogliati C, Torzillo D, Villa AM, Egidi AM, Repetto EC, Ridolfo AL, Corbellino M, Galli M. Chagas disease knocks on our door: a cross‐sectional study among Latin American immigrants in Milan. Italy Clin Microbiol Infect 2018; 24: 1340. [DOI] [PubMed] [Google Scholar]
- 3. Bern C. Chagas' disease. N Engl J Med 2015; 373: 456–466. [DOI] [PubMed] [Google Scholar]
- 4. Bocchi EA, Bestetti RB, Scanavacca MI, Cunha Neto E, Issa VS. Chronic Chagas heart disease management: from etiology to cardiomyopathy treatment. J Am Coll Cardiol 2017; 70: 1510–1524. [DOI] [PubMed] [Google Scholar]
- 5. Shen L, Ramires F, Martinez F, Bodanese LC, Echeverría LE, Gómez EA, Abraham WT, Dickstein K, Køber L, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Jhund PS, Gimpelewicz CR, McMurray JJV, PARADIGM‐HF and ATMOSPHERE Investigators and Committees . Contemporary characteristics and outcomes in chagasic heart failure compared with other nonischemic and ischemic cardiomyopathy. Circ Heart Fail 2017; 10: pii: e004361. [DOI] [PubMed] [Google Scholar]
- 6. Espinosa R, Carrasco HA, Belandria F, Fuenmayor AM, Molina C, González R, Martínez O. Life expectancy analysis in patients with Chagas' disease: prognosis after one decade (1973–1983). Int J Cardiol 1985; 8: 45–56. [DOI] [PubMed] [Google Scholar]
- 7. Mady C, Cardoso RH, Barretto AC, da Luz PL, Bellotti G, Pileggi F. Survival and predictors of survival in patients with congestive heart failure due to Chagas' cardiomyopathy. Circulation 1994; 90: 3098–3102. [DOI] [PubMed] [Google Scholar]
- 8. Theodoropoulos TAD, Bestetti RB, Otaviano AP, Cordeiro JA, Rodrigues VC, Silva AC. Predictors of all‐cause mortality in chronic Chagas' heart disease in the current era of heart failure therapy. Int J Cardiol 2008; 128: 22–29. [DOI] [PubMed] [Google Scholar]
- 9. Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher‐Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med 2006; 355: 799–808. [DOI] [PubMed] [Google Scholar]
- 10. Ayub‐Ferreira SM, Mangini S, Issa VS, Cruz FD, Bacal F, Guimarães GV, Chizzola PR, Conceição‐Souza GE, Marcondes‐Braga FG, Bocchi EA. Mode of death on Chagas heart disease: comparison with other etiologies. A subanalysis of the REMADHE prospective trial. PLoS Negl Trop Dis 2013; 25: e2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiorelli AI, Santos RH, Oliveira JL Jr, Lourenço‐Filho DD, Dias RR, Oliveira AS, da Silva MF, Ayoub FL, Bacal F, Souza GE, Bocchi EA, Stolf NA. Heart transplantation in 107 cases of Chagas' disease. Transplant Proc 2011; 43: 220–224. [DOI] [PubMed] [Google Scholar]
- 12. Issa VS, Amaral AF, Cruz FD, Ferreira SM, Guimarães GV, Chizzola PR, Souza GE, Bacal F, Bocchi EA. Beta‐blocker therapy and mortality of patients with Chagas cardiomyopathy: a subanalysis of the REMADHE prospective trial. Circ Heart Fail 2010; 3: 82–88. [DOI] [PubMed] [Google Scholar]
- 13. Mocelin AO, Issa VS, Bacal F, Guimarães GV, Cunha E, Bocchi EA. The influence of aetiology on inflammatory and neurohumoral activation in patients with severe heart failure: a prospective study comparing Chagas' heart disease and idiopathic dilated cardiomyopathy. Eur J Heart Fail 2005; 7: 869–873. [DOI] [PubMed] [Google Scholar]
- 14. de Macedo IS, Dinardi LFL, Pereira TV, de Almeida LKR, Barbosa TS, Benvenuti LA, Ayub‐Ferreira SM, Bocchi EA, Issa VS. Thromboembolic findings in patients with heart failure at autopsy. Cardiovasc Pathol 2018; 35: 23–28. [DOI] [PubMed] [Google Scholar]
- 15. Terhoch CB, Moreira HF, Ayub‐Ferreira SM, Conceição‐Souza GE, Salemi VMC, Chizzola PR, Oliveira MT Jr, Lage SHG, Bocchi EA, Issa VS. Clinical findings and prognosis of patients hospitalized for acute decompensated heart failure: analysis of the influence of Chagas etiology and ventricular function. PLoS Negl Trop Dis 2018; 12: e0006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J, ADHERE Scientific Advisory Committee and Investigators , ADHERE Study Group . In‐hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol 2005; 46: 57–64. [DOI] [PubMed] [Google Scholar]
- 17. Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic PM, Drozdz J, Ferrari R, Anker S, Coats A, Filippatos G, Crespo‐Leiro MG, Mebazaa A, Piepoli MF, Maggioni AP, Tavazzi L, ESC‐HFA HF Long‐Term Registry investigators . In‐hospital and 1‐year mortality associated with diabetes in patients with acute heart failure: results from the ESC‐HFA Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 54–65. [DOI] [PubMed] [Google Scholar]
- 18. Albuquerque DC, Neto JD, Bacal F, Rohde LE, Bernardez‐Pereira S, Berwanger O, Almeida DR, Investigadores Estudo BREATHE . I Brazilian Registry of Heart Failure—clinical aspects, care quality and hospitalization outcomes. Arq Bras Cardiol 2015; 104: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P, Interamerican Society of Cardiology . The reality of heart failure in Latin America. J Am Coll Cardiol 2013; 62: 949–958. [DOI] [PubMed] [Google Scholar]
- 20. Bestetti RB, Otaviano AP, Fantini JP, Cardinalli‐Neto A, Nakazone MA, Nogueira PR. Prognosis of patients with chronic systolic heart failure: Chagas disease versus systemic arterial hypertension. Int J Cardiol 2013; 168: 2990–2991. [DOI] [PubMed] [Google Scholar]
- 21. Santos LN, Rocha MS, Oliveira EN, Moura CA, Araujo AJ, Gusmão ÍM, Feitosa‐Filho GS, Cruz CM. Decompensated chagasic heart failure versus non‐chagasic heart failure at a tertiary care hospital: clinical characteristics and outcomes. Rev Assoc Med Bras 2017; 63: 57–63. [DOI] [PubMed] [Google Scholar]
- 22. Shore S, Grau‐Sepulveda MV, Bhatt DL, Heidenreich PA, Eapen ZJ, Hernandez AF, Yancy CW, Fonarow GC. Characteristics, treatments, and outcomes of hospitalized heart failure patients stratified by etiologies of cardiomyopathy. JACC Heart Fail 2015; 3: 906–916. [DOI] [PubMed] [Google Scholar]
- 23. Ostręga M, Gierlotka MJ, Słonka G, Nadziakiewicz P, Gąsior M. Clinical characteristics, treatment, and prognosis of patients with ischemic and nonischemic acute severe heart failure. Analysis of data from the COMMIT AHF registry. Pol Arch Intern Med 2017; 127: 328–335. [DOI] [PubMed] [Google Scholar]
- 24. Lourenço C, Saraiva F, Martins H, Baptista R, Costa S, Coelho L, Vieira H, Monteiro P, Franco F, Gonçalves L, Providência LA. Ischemic versus non‐ischemic cardiomyopathy—are there differences in prognosis? Experience of an advanced heart failure center. Rev Port Cardiol 2011; 30: 181–197. [PubMed] [Google Scholar]
- 25. Ochiai ME, Cardoso JN, Vieira KR, Lima MV, Brancalhao EC, Barretto AC. Predictors of low cardiac output in decompensated severe heart failure. Clinics 2011; 66: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 2012; 9: 576–589. [DOI] [PubMed] [Google Scholar]
- 27. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 28. McCallum W, Tighiouart H, Kiernan MS, Huggins GS, Sarnak MJ. Relation of kidney function decline and NT‐proBNP with risk of mortality and readmission in acute decompensated heart failure. Am J Med 2019;pii: S0002–9343(19)30529–7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Higuchi LM, Lopes EA, Barretto AC, Stolf N, Bellotti G, Verginelli G, Pileggi F. Endomyocardial biopsy of the right ventricle. Significance of the ultrastructural changes in Chagas' cardiopathy. Arq Bras Cardiol 1985; 44: 171–175. [PubMed] [Google Scholar]
- 30. Moreira HT, Volpe GJ, Marin‐Neto JA, Ambale‐Venkatesh B, Nwabuo CC, Trad HS, Romano MM, Pazin‐Filho A, Maciel BC, Lima JA, Schmidt A. Evaluation of right ventricular systolic function in Chagas disease using cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2017; 10: e005571. [DOI] [PubMed] [Google Scholar]
- 31. Moreira HT, Volpe GJ, Marin‐Neto JA, Nwabuo CC, Ambale‐Venkatesh B, Gali LG, Almeida‐Filho OC, Romano MMD, Pazin‐Filho A, Maciel BC, Lima JAC, Schmidt A. Right ventricular systolic dysfunction in Chagas disease defined by speckle‐tracking echocardiography: a comparative study with cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2017; 30: 493–502. [DOI] [PubMed] [Google Scholar]
- 32. Ardito SQ, Bestetti RB, Cardinalli‐Neto A, Otaviano AP, Nogueira PR. Chronic renal impairment in patients with Chagas cardiomyopathy with chronic systolic heart failure: prevalence and prognostic significance. Int J Cardiol 2011; 152: 133–134. [DOI] [PubMed] [Google Scholar]
- 33. Ventura‐Garcia L, Roura M, Pell C, Posada E, Gascón J, Aldasoro E, Muñoz J, Pool R. Socio‐cultural aspects of Chagas disease: a systematic review of qualitative research. PLoS Negl Trop Dis 2013; 7: e2410. [DOI] [PMC free article] [PubMed] [Google Scholar]


