Figure 3.
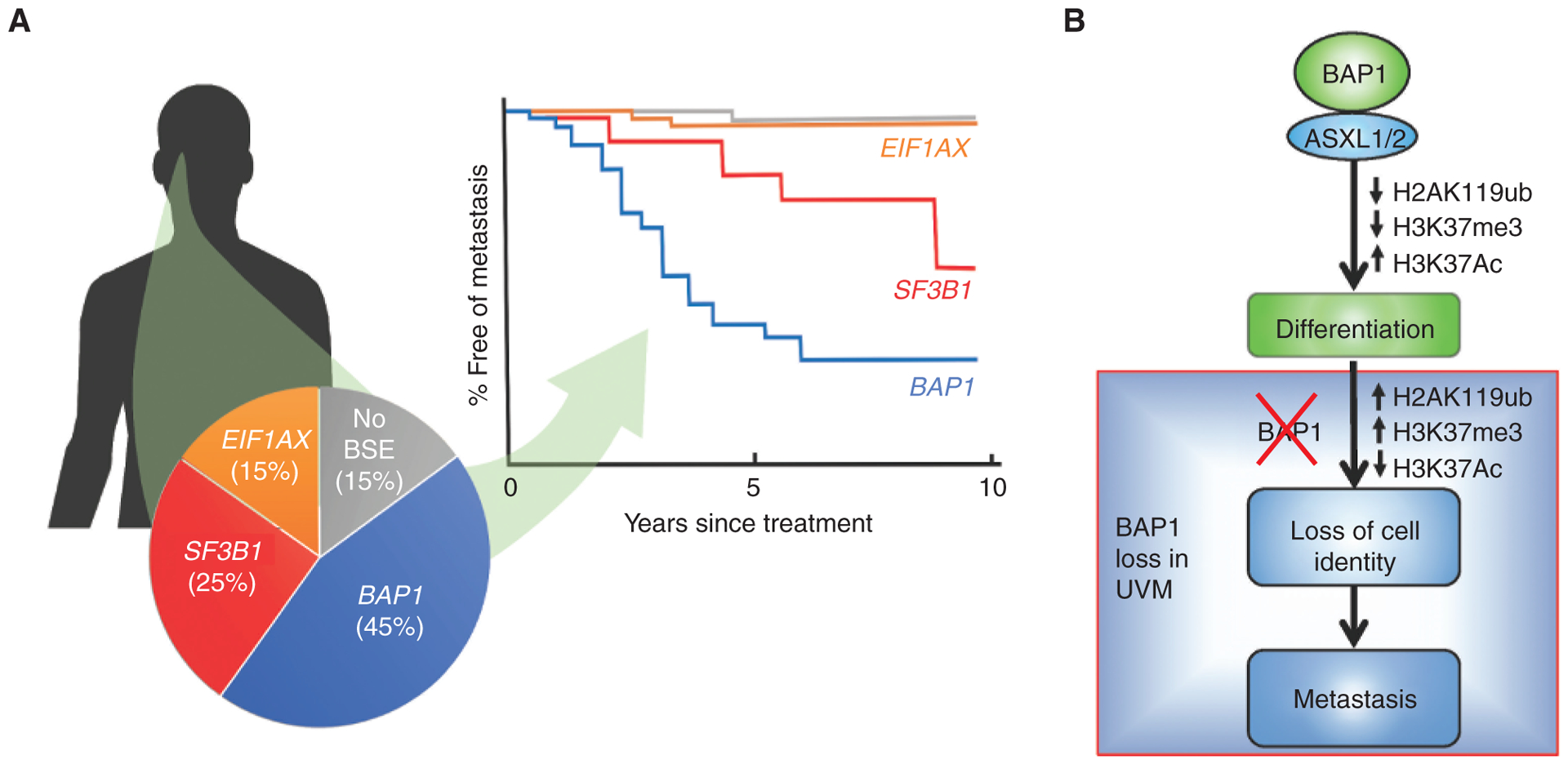
BAP1 mutations in uveal melanoma (UVM). A, Uveal melanomas arise in the iris, ciliary body, and choroid of the uveal tract of the eye. Their metastatic potential is determined by mutually exclusive “BSE” progression mutations in BAP1, SF3B1 (and rarely in other splicing factors), and EIF1AX. Inactivating mutations in BAP1, when coupled with the loss of the other copy of chromosome 3, result in high metastatic risk associated with the class 2 gene-expression profile. Hemizygous mutations in SF3B1 (or rarely in other splicing factors) retain the class 1 gene expression profile and are associated with intermediate metastatic risk. Hemizygous mutations in the translation initiation factor EIF1AX also retain the class 1 gene expression profile and are associated with low metastatic risk. Uveal melanomas without BSE mutations have a prognosis similar to those with EIF1AX mutations. This figure represents a synopsis of published data (68, 101, 178). B, Recent work indicates that BAP1 regulates the switch from progenitor to differentiated cell types in vertebrate development, not only through effects on H2A ubiquitination but perhaps more importantly by repressing HDACs and allowing acetylation of H3K27 to activate genes involved in differentiation in neural crest and other lineages. Loss of BAP1 abrogates this differentiation switch in development that parallels phenotypic and transcriptomic alterations observed in association with BAP1 mutation in uveal melanoma (114).
