Abstract
Introduction
Youth with brain-based disabilities (BBDs), as well as their parents/caregivers, often feel ill-prepared for the transfer from paediatric to adult healthcare services. To address this pressing issue, we developed the MyREADY TransitionTM BBD App, a patient-facing e-health intervention. The primary aim of this randomised controlled trial (RCT) was to determine whether the App will result in greater transition readiness compared with usual care for youth with BBD. Secondary aims included exploring the contextual experiences of youth using the App, as well as the interactive processes of youth, their parents/caregivers and healthcare providers around use of the intervention.
Methods and analysis
We aimed to randomise 264 youth with BBD between 15 and 17 years of age, to receive existing services/usual care (control group) or to receive usual care along with the App (intervention group). Our recruitment strategy includes remote and virtual options in response to the current requirements for physical distancing due to the COVID-19 pandemic. We will use an embedded experimental model design which involves embedding a qualitative study within a RCT. The Transition Readiness Assessment Questionnaire will be administered as the primary outcome measure. Analysis of covariance will be used to compare change in the two groups on the primary outcome measure; analysis will be intention-to-treat. Interviews will be conducted with subsets of youth in the intervention group, as well as parents/caregivers and healthcare providers.
Ethics and dissemination
The study has been approved by the research ethics board of each participating site in four different regions in Canada. We will leverage our patient and family partnerships to find novel dissemination strategies. Study findings will be shared with the academic and stakeholder community, including dissemination of teaching and training tools through patient associations, and patient and family advocacy groups.
Trial registration number
Keywords: clinical trials, developmental neurology & neurodisability, rehabilitation medicine
Strengths and limitations of this study.
This study takes a user-centred approach, using a patient-facing e-health intervention to engage with youth and improve transition readiness for individuals with brain-based disabilities (BBDs) transitioning to adult healthcare.
The embedded mixed-method randomised controlled trial design is ideal to answer complex research questions and provides stronger and richer evidence than a single method alone.
The generalisability of the study findings may be limited to youth with BBD who have started to take charge of their own health.
Prior to study recruitment, we proactively adapted our study methods in response to the COVID-19 pandemic.
Strong patient and family involvement at all stages of the study focuses on improving the lived experiences of youth with BBD and their families.
Introduction
With healthcare innovations, more and more people with paediatric-onset disabilities are surviving into adulthood, thus increasing the need for proactive care strategies for this growing cohort.1 The process of transition to adulthood can be challenging, as youth with disabilities and their families move from familiar paediatric healthcare services and learn how to navigate new adult services. In many jurisdictions in Canada, as elsewhere, the transfer from paediatric to adult healthcare services is set by policy and occurs regardless of whether the youth is ready for transition of care. Poor healthcare transition can have negative health outcomes and can result in poor quality of life for youth with brain-based disabilities (BBDs), such as autism spectrum disorder, cerebral palsy, epilepsy, fetal alcohol spectrum disorder and spina bifida. Lack of access to healthcare services can result in increased use of high-cost healthcare services, increased emergency department visits, family burden and exacerbated health issues.2–5
In Canada, this transfer of care typically occurs at age 18. Since the policy-driven age of transfer cannot be changed, we designed the Readiness in Youth for Transition Out of Pediatric Care (READYorNotTM) Brain-Based Disabilities Trial to evaluate a patient-facing e-health intervention (MyREADY TransitionTM BBD App) aimed at fostering self-management and self-advocacy skills in youth with BBD to improve their readiness for adult healthcare.
Youth with BBD are expected to be prepared for healthcare transfer by developing the knowledge and skills to manage their health condition. Transition is defined as ‘a purposeful, planned process that addresses the medical, psychosocial and educational/vocational needs of adolescents and young adults with chronic physical and medical conditions as they move from child-centered to adult-oriented health care systems’.6 The field of transition of care has grown over the past few decades, with several calls to action to improve processes of transition and to develop interventions and resources with a vision to maximise lifelong functioning through uninterrupted healthcare services as individuals move from adolescence to adulthood.7–9
The use of information technology, such as eHealth interventions and applications (‘Apps’), is an appealing, accessible and flexible way to engage youth with BBD and their families. Patient engagement via information technology has been shown to directly impact patient behaviour in a way that promotes positive health outcomes, patient satisfaction and care delivery efficiency; reduces costs; and improves quality of care and patient safety.10 11 We developed the MyREADY TransitionTM BBD App to improve transition readiness of youth with BBD. This App was co-created with researchers, healthcare professionals, technology designers, youth and families working in partnership.
Objectives
The aim of this study was to determine whether the MyREADY TransitionTM BBD App intervention will result in greater transition readiness compared with usual care for youth with BBD between 15 and 17 years of age. We hypothesise that youth who receive their usual care and use the MyREADY TransitionTM BBD App intervention will have higher self-management change scores over a 6-month period compared with youth who receive their usual care. The secondary aim of the study included exploring the contextual experiences of youth using the App, as well as the interactive processes of youth, their parents/caregivers and healthcare providers around use of the intervention. We also aimed to explore health economic outcomes by comparing the incremental cost of the intervention with current standard of care per unit of effectiveness.
Design
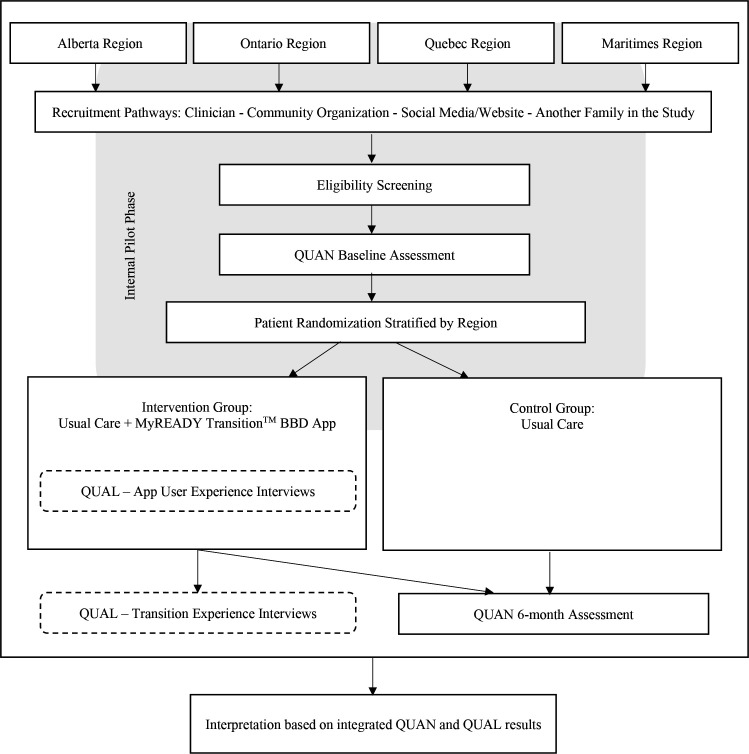
This protocol paper describes a randomised controlled trial (RCT) using a mixed-method study design. The RCT will use a pragmatic approach to test whether the intervention works under usual conditions. Specifically, we will use an embedded experimental model design12 which will involve embedding a qualitative component within the RCT (see figure 1). Our selection of outcome metrics and the comprehensive design to evaluate this intervention was guided by the Institute for Healthcare Improvement’s Triple Aim framework (better health, better experience at lower cost).
Figure 1.
Study design.QUAN, quantitative; QUAL, qualitative.
Patient and public involvement
The Canadian Institutes of Health Research’s Strategy for Patient-Oriented Research endorses that patients and parents/caregivers with ‘lived experiences’, together with health professionals and decision makers, join researchers as members of the research team. Since the very beginning, we have engaged a core group of youth, parents/caregivers and healthcare stakeholders, including a Patient and Family Advisory Council (PFAC), composed of representative youth (adolescents and young adults) and parents/caregivers. We designed our study in collaboration with patients and families and healthcare professionals. Our partnership with the PFAC will continue throughout the execution of our RCT, thus enhancing participant recruitment, data collection, engagement planning, and knowledge translation (KT). In the KT stage of the project, we will work together on novel and meaningful ways to share the study findings about potential benefits of the App.
Methods
Study setting
The READYorNotTM Brain-Based Disabilities Trial is a patient-oriented project of Child Health Initiatives Limiting Disability–Brain Research Improving Growth and Health Trajectories (CHILD-BRIGHT), which is a pan-Canadian research programme. A large cohort of youth with BBD living in Alberta, Ontario, Quebec and the Maritimes in Canada will be recruited.
Eligibility criteria
The following are study inclusion criteria: (1) age of 15–17 years; (2) a diagnosis of autism spectrum disorder, cerebral palsy, epilepsy, fetal alcohol spectrum disorder or spina bifida; (3) in paediatric care in one of the four study regions and for whom a discharge from paediatric care is planned but not for at least 6 months; (4) cognitive ability to provide informed assent and the ability to read and understand English or French; (5) access to the internet and a smartphone, iPad/tablet or desktop computer; and (6) TRANSITION-Q13 score of >40 (as a screen to define a minimum threshold for transition readiness based on our earlier work). In a validation sample of Ontario youth aged 12–25 years with chronic health conditions, including our target population, the average TRANSITION-Q scores were 40 for 13 year olds, 53 for 15 year olds and 59 for 17 year olds.14 The decision to set the threshold at >40 aligns with clinical judgement and is conservative, given that the youngest age group in the validation sample demonstrated this minimum level of readiness. The TRANSITION-Q screen will inherently not include youth who have severe intellectual disability, and/or those who rely significantly on parents/caregivers in most areas of daily functioning, self-care and/or communication.
Individuals will be excluded if they are (1) in ‘acute crisis’ with unstable physical or mental health that would interfere with the ability to participate in the study; (2) have sensory impairments, such as uncorrected vision or hearing loss, which would interfere with the use of the App; or (3) are enrolled in a potentially confounding trial (e.g., a different transition intervention study).
Intervention
The MyREADY TransitionTM BBD App is designed to educate and empower youth with BBD as they prepare for transitioning from paediatric to adult care. We collaborated with the PFAC to inform the design and help tailor the App to incorporate features that were meaningful to them. The App is constructed as a ‘journey in the city’ with a mentor (virtual coach) that helps the user navigate the buildings and sequentially introduces the educational sections. The content of the App is composed of messages, texts, quizzes, videos and skill-based-achievement challenges. The App uses pop-up features to manage reminders aimed at keeping engagement and ensuring adherence to the App. The App has 19 visits organised into five ordered chapters. In terms of exposure time, there is ‘planned’ flexibility to allow participants to proceed through the MyREADY TransitionTM BBD App intervention at their own pace. There is a timer in the App to inform participants when the next visit is unlocked. Participants will receive instructions to wait at least 1 day between visits. This waiting period will allow participants to process and reflect on the take-away messages in each visit and/or engage in one of the suggested between-session practice activities. The waiting period will also moderate the pace and aligns with how young people learn and digest information. There are approximately 5–7 hours of content within the App. For the RCT, the recommended exposure to the App intervention is between one visit per day (19 days) and one visit per week (19 weeks). Games and fun activities are incorporated to encourage youth to explore the App between visits.
For participants randomised into the intervention group, the research assistant (RA) will help them download the App on their device. To ensure that the participant understands how to access the App’s features, they will watch an introduction video demonstrating the first visit of the App, and they will be given a reference handout with tips and strategies for using the App. Participants will also receive a website link for App support, including support for download, access to the introduction video, a list of frequently asked questions, as well as a series of short how-to tutorial videos about different features in the App. The RCT research coordinator and research information technology team will further support the use of the App and troubleshoot issues as they arise. This will be done using a designated email to capture and respond to queries and will include an automated response, indicating receipt and approximate response time. During study participation, participants will receive reminders to promote the use of the MyREADY TransitionTM BBD App. Parents of youth in the intervention group and healthcare providers in recruiting clinics will receive guidelines on how to support youth in the intervention group, including an explanation that we want to know how youth are using the App independently, suggestions about ways they can help youth use the App without influencing their use of the App and information about how youth can access App support.
Usual care (intervention and control groups)
Participants randomised into the control group will continue to get the same care they have been getting (their usual care). Youth participants in both the control and intervention groups, along with their parent/caregiver, will receive a standard reference handout (online supplemental file 1) that will provide a basic overview of what they might expect as they get ready for transition. This handout ensures that all study participants have a minimum standard of preparation beyond the usual care they are receiving. Any support the youth (and parents/caregivers) receive as part of any ongoing transition programmes in usual care will be documented by youth, parents/caregivers and healthcare providers. Documentation will include an inventory checklist with a section to add specific information relevant to the transition process. Participants and their parents/caregivers will also be asked what they perceive as supports and if they received these supports.
bmjopen-2021-048756supp001.pdf (860.4KB, pdf)
Outcomes
In this RCT, both quantitative and qualitative data types will be collected. All data collection forms will be available in both English and French. Detailed demographic information from parents/caregivers will be collected to understand and describe the different kinds of families participating in CHILD-BRIGHT studies and to compare this study sample to youth with BBD across Canada.
Measures
The primary outcome is transition readiness, which will be measured with the 29-item version of the Transition Readiness Assessment Questionnaire (TRAQ).15 While TRAQ measure refinement is ongoing and other versions are now available, our sample size calculation is based on findings from an intervention trial16 where the 29-item version of the TRAQ was used. The 29-item version has a self-management domain (16 items) and a self-advocacy domain (13 items). Each item is scored from 1 to 5, where 1=‘I do not need to do this’, 2=‘I do not know how but I want to learn’, 3=‘I am learning to do this’, 4=‘I have started doing this’ and 5=‘I always do this when I need to’. It is a validated, patient-centred questionnaire used in previous studies to measure transition readiness17 and is designed to be self-administered at baseline and 6 months. Details about the TRAQ and other secondary outcome measures are provided in online supplemental file 2).
bmjopen-2021-048756supp002.pdf (153.4KB, pdf)
Secondary outcomes will evaluate whether the intervention has an effect on population health. All youth will be asked to complete the Canadian Occupational Performance Measure,18 TRANSITION-Q13 and PedsQLTM Paediatric Quality of Life Instrument, Generic Core and Teen Report (13–18 years).19 To assess the impact of the App on the families’ healthcare experience, parents/caregivers will complete the Measure of Process of Care (family-centred care),20 and youth will be asked to complete the Newest Vital Sign21 as a measure of their health literacy at baseline. Cost utility and cost-effectiveness will be measured using Health Utilities Index® (HUI2/3) Proxy-Assessed (health-related quality of life)22 and Resource Use Questionnaire.23 In addition, consent will be requested to obtain youth participants’ health card numbers to link information provided in the study with provincial health administrative data about use of health services, such as physician office visits, visits to emergency rooms and hospitalisations during the study period. Participants may choose to participate without sharing their health card information.
The MyREADY TransitionTM BBD App will log user metrics which will be useful during the intervention (to monitor App use and provide support) and during the evaluation phases of the project (to quantify App use). The App will collect data related to end user login to the system (times and dates), visit/session completion and average time spent, number of clicks by challenge and event type, access to games at the arcade, and time spent on a visit/session and trends over time. Within the App, users are asked to provide feedback at the end of each visit. They will rate their experience on a 3-point scale using emojis for happy, neutral and unhappy/sad. Questions ask about experiences with the videos, quizzes and challenges, and the usefulness of the content in the visit/session and will ask whether they completed the visit/session alone or with help. The App will collect demographic data provided during the registration process and device information, such as the type of device and its operating system. Participants in the intervention group will also be asked to complete the System Usability Scale24 at the 6-month visit. The measure will focus on users’ use of the App and its features, the perceived value, experience and satisfaction with the intervention.
Interviews
As part of the embedded qualitative study (figure 1), we will purposefully sample two subsets of participants for different study aims from the sample of 132 youth participants in the intervention group. All interviews will be one-on-one, semistructured, conducted over the phone or by videoconference (e.g., Zoom), and will be audio-recorded.
A subset of approximately 30 youth participants, as well as 10 parents/caregivers and 10 healthcare providers, will be interviewed to describe and understand the primary outcomes after the 6-month quantitative data collection point. These interviews will be conducted following their completion of the RCT outcome measures. The purpose will be to understand youth perceptions of their transition readiness skills and awareness after using the App, to understand how they may have used the App in their everyday lives and in interactions with healthcare providers, and to understand how the App might have influenced their care. Parent and healthcare providers will be invited to share their perspectives on the potential of the App to improve transition readiness.
To address our secondary aim, a subset of approximately 20 youth participants will be interviewed to capture process and user experience of the App at the end of exposure or after 6 months, whichever timepoint comes first. These interviews will complement quantitative findings related to App use, and for opportunities to improve the App. Youth will be asked how the App was used initially and over time, barriers and facilitators to its use, and participants’ experience and satisfaction with the App and its features.
Sample size
The primary sample will be composed of 264 youth with BBD, aged 15–17 years, who are recruited in one of the four study regions (Alberta, Ontario, Quebec and Maritimes) and who are at least 6 months pretransfer to the adult healthcare system. The parent or caregiver of the youth will also be recruited to the study. The study aims to have an equal number of participants in the control and intervention groups. Randomisation will be stratified by region with a 1:1 allocation ratio for patients: 132 in the control group (continuing with usual care) and 132 in the intervention group (continuing with usual care and receiving App intervention). The primary outcome measure (TRAQ) has been validated on a sample of Canadian youth with congenital heart disease16 and, in the absence of literature specific to BBD, our sample size calculation was based on these findings: we anticipate a mean TRAQ self-management baseline score of 3.01 (SD 1.02) (out of a possible 5.0) as reported for youth (with congenital heart disease)16 without a transition intervention and an anticipated mean score of 4.0 at 6 months of follow-up, resulting in a change score of 1 (ie, 1 SD), with a=0.05% and 90% power. Therefore, in each region, 23 youth are required in each of the two arms×four regions=184. With an estimated attrition rate of 30%, we will enrol a total of 264 participants across the four regions.
Recruitment
Before the onset of the study, we expanded our recruitment approach (online supplemental file 3) to include strategies to facilitate the study’s operations within the context of physical distancing measures related to the COVID-19 pandemic.25 26 First, clinicians in the patient’s circle of care at each clinic site in each of the four regions will approach eligible participants to ascertain interest and obtain permission for the RA to contact them. They may obtain this permission in person after physical distancing measures are lifted, by telephone or by mail. Recruitment materials will also be shared on websites and social media. As a result, some participants may wish to self-refer and contact the RA directly. The RAs will contact potential participants by phone to complete a screening checklist and confirm eligibility. If there is a clinic appointment scheduled, contact will be made in advance so that they have an opportunity to learn about the study and ask questions. The consent and assent form will be sent to potential participants in advance of the scheduled visit. Due to physical distancing measures, individuals can choose to have the forms sent to them by email or by mail. Prior to collecting any data, informed consent/assent will be obtained. All youth will provide assent and a parent/caregiver will also provide consent both for their child and for themselves. Again, to accommodate physical distancing measures, we have added a telephone verbal consent procedure. Ideally, participants will complete the baseline study visit at the same appointment time. If the latter is not possible, the RA will arrange another study appointment convenient for both the youth and parent/caregiver.
bmjopen-2021-048756supp003.pdf (258.4KB, pdf)
Randomisation
Randomisation will be stratified by region with a 1:1 allocation ratio for participants: control group, who will continue with usual care, or intervention group, who will continue with usual care and receive the MyREADY TransitionTM BBD App. The unit of randomisation is the patient. Variable block randomisation will be used with block sizes of 2, 4, 6 and 8. Allocation will be done via Research Electronic Data Capture (REDCap®).27 Individuals who meet the eligibility requirements at the point of screening and who give consent to participate will be randomised after the baseline questionnaires are completed. Participants allocated to the control or usual care group will not know the specific details of the electronic intervention being offered to the intervention group. Due to the nature of the intervention, participants cannot be blinded to group allocation; however, outcome assessment and data analysis will be blinded.
Procedures
Prior to the start of the study, each study RA will complete an e-learning module training provided by the research team (online supplemental file 4). Procedural fidelity will be monitored.
bmjopen-2021-048756supp004.pdf (71.8KB, pdf)
Data collection
To track participants according to the Consolidated Standards of Reporting Trials guidelines,28 29 a deidentified log of screened youth patients at all participating sites will be kept, recording inclusion/exclusion criteria and reasons for eligible youth patients not being recruited or randomised.
In case the baseline visit is not done in person due to physical distancing measures, we have added the option to conduct the baseline visit via telephone or ‘Zoom’ meeting (Zoom Video Communications, Inc.). To better establish rapport with participants, the RA may conduct the Zoom meeting with their own camera on.30 31 Participants will have the option to turn on their camera or keep it off. Zoom meetings will not be recorded.
A data transfer agreement will be in place with each participating centre to ensure secure transfer and storage of the study data. The RCT will be centrally managed by the RCT coordinator at McMaster University’s CanChild Centre for Childhood Disability Research. Research files will be stored on the CanChild Active Directory at McMaster, on a secure network that is in a tier three data facility. The CanChild Active Directory is a firewall-protected server to which only the principal investigators and research coordinators will have access. Remote access to the CanChild directory is via VPN. Any personal information collected will be entered into password-protected SPSS IBM Statistics V.26 or Excel V.16.41 files and stored on the CanChild Active Directory, separate from other study data. Qualitative data will be stored on the CanChild Active Directory and managed electronically using NVivo, a qualitative data analysis software system. Research staff will password protect their electronic and audio digital files from the interview sessions and can transmit these into the secure cloud storage provided through McMaster’s MacDrop (https://drop.mcmaster.ca), with final storage on the CanChild Active Directory. There will be a code linking identifiers to the study participant. The study doctors, regional coordinator and study RAs at each local recruiting site will have access to the key linking study identification number with participant identity for the participants at that site.
REDCap® 27 is a secure web application for building and managing online surveys and databases (www.project-redcap.org). REDCap® questionnaire data for this project will be hosted by the Department of Paediatrics at McMaster University. Deidentified data are stored on a secure, firewall protected server with regular backup in the Faculty of Health Sciences Computer Services Unit with only the https port available to the internet. Data can be entered into REDCap® 27 by designated users or survey respondents from any computer with an internet connection. Surveys will be completed on paper by a study participant and entered into the REDCap® form by the RA at the recruitment site or completed online by a study participant directly into the REDCap® form. Since no identifying information is stored in REDCap®, the link to electronic survey forms will be sent to the RA and the RA will email it to the participant.
Internal pilot phase
The British Medical Research Council explicitly recommends the use of feasibility studies prior to phase III clinical trials.32 To guide the planning and to enhance the likelihood of success of our full-scale RCT, the first 3 months of recruitment will comprise an internal pilot phase where study procedures will be observed and considerations will be taken into account about key implementation aspects, such as recruitment (including refusal rates and screening process), multisite coordination/collaboration (including communication, documentation and provision of support) and intervention uptake and adherence (including technical support needs among App users). The results will be used to refine and enhance the research design. The RCT will proceed with procedural modifications based on the findings of the internal pilot study and final study analyses will incorporate all data. As long as the alpha level is controlled, internal pilot designs have, at most, a small adverse effect on the significance level and may greatly improve the power.33
Analysis
A mixed-method approach will be used that combines both quantitative and qualitative research methods and techniques. This methodology is commonly used in patient-centred care research as both qualitative and quantitative methods combined can serve to answer complex research questions and allow for stronger and richer evidence than could be accomplished by a single method alone. Contextual qualitative data are necessary where the complexity of different sites throughout Canada might create challenges for evaluating the effectiveness of the intervention. This mixed-method approach will allow us to shed light on any potential variations in effects emerging from the RCT.
Quantitative analysis
Patient demographics and baseline outcome variables will be summarised using descriptive summary measures. Analyses will be performed using SAS V.9.4 (Cary, North Carolina, USA). Intention-to-treat analysis will be used. We will use multiple imputation to handle missing data. Analysis of covariance will be used to adjust for baseline function as a sensitivity analysis to address any residual imbalance from the randomisation. Since participants will be recruited from only four regions, we will model the effect of region as a fixed effect rather than a random effect.34 A fixed effects model will allow region-specific intervention effects to be modelled (ie, region by intervention interaction effects). If no region by intervention interactions are found, the interaction terms will be dropped from the model and we will estimate an overall intervention effect. Detailed information about the statistical analysis addressing each objective is provided in online supplemental file 5).
bmjopen-2021-048756supp005.pdf (27KB, pdf)
Cost-effectiveness analyses will be conducted from the patient and system (provincial) perspective. Costing relates to the cost to develop and resources to support the intervention as well as resources used for treatment/management of participants’ health conditions over the study. We will use an exploratory health economic evaluation to assess youth engagement prior to transition. A decision tree that models the intervention and control groups will be conducted. A 3% discount rate will be applied to outcomes and costs extending beyond 1 year. All measurement and analytic assumptions made for the base case analysis will be clearly stated. The mean cost per child and the mean effectiveness result per child for each group will be represented in an incremental cost-effectiveness ratio, the ratio of the difference between groups in mean cost per patient to the difference in mean effectiveness. Subgroups of patients based on baseline demographic factors may be analysed separately, if appropriate. Extensive sensitivity analysis including probabilistic sensitivity analysis will be undertaken to test the robustness of the results to variations in underlying assumptions.
Qualitative data analysis
Individual interviews will be audio-recorded and transcribed verbatim. Data will be stored and managed electronically using NVivo V.11. Conventional content analysis35 will be used to code, categorise and synthesise the data to contextualise the analysis of the primary aim of the RCT. In addition, data related to usability of the App will be monitored and analysed to inform ongoing App development.
In summary, readiness for healthcare transition means that youth with BBD need to develop the necessary skills to manage their health condition. There are real gaps in empowerment and education for this population at this crucial stage in their life. The CHILD-BRIGHT READYorNotTM Brain-Based Disabilities Trial is a mixed-method RCT to test a novel patient-faced e-Health intervention. While the App is an educational tool for youth with BBD to take charge of their health, there is an animated ‘mentor’ character in the App who serves as a guide to support youth as they learn the necessary skills for their journey through healthcare transition. Youth with BBD from four regions in Canada are participating in a study designed in collaboration with patients and families to ensure that findings are relevant, meaningful and applicable to the lives of people receiving transition care. Our recruitment strategy includes remote and virtual options in response to the current requirements for physical distancing due to the COVID-19 pandemic. We expect that these novel strategies will continue to be beneficial even after the physical distancing measures are relaxed, and that the societal trend toward telehealth solutions in healthcare may enhance future uptake of the intervention into clinical practice.36
Ethics and dissemination
The study has been approved by the Research Ethics Board of each participating site (online supplemental file 6): Hamilton Integrated Research Ethics Board (Clinical Trials Ontario #1666), (Alberta) Health Research Ethics Board (Health Panel #MS2_Pro00086027), Horizon Health Network Research Ethics Board (#2018–2689), Research Ethics Board at the University of New Brunswick (Saint John #037–2019), Mount Allison University Research Ethics Board (#102606) and IWK Research Ethics Board (#1 025 247). The study will be conducted according to the principles of the Declaration of Helsinki. Findings of the RCT will be published in open access, peer-reviewed scientific journals and presented at national and international conferences. KT activities directed at the stakeholder community will also include presentations at meetings, and dissemination of teaching and training tools through patient associations, and patient and family advocacy groups. All participants will receive a plain language report at the end of the study after the RCT results have been analysed. After the completion of this RCT, our team will explore the potential to make the App more widely available.
bmjopen-2021-048756supp006.pdf (69.2KB, pdf)
Supplementary Material
Footnotes
Twitter: @Dr_Gorter, @AdrienneK_PhD, @lindaa728, @aly_vdl
Correction notice: Three contributors were added to the study group.
Collaborators: The Child Health Initiatives Limiting Disability–Brain Research Improving Growth and Health Trajectories (CHILD-BRIGHT) Readiness in Youth for Transition Out of Pediatric Care Brain-Based Disabilities Trial Study Group. Core team: Dr Jan Willem Gorter, CanChild Centre for Childhood Disability Research, Department of Pediatrics, McMaster University, McMaster Children’s Hospital, Hamilton, Ontario, Canada (nominated principal investigator (PI)); Dr Ariane Marelli, McGill University and McGill University Health Centre, Montreal, Quebec, Canada (co-PI); Dr Adrienne Kovacs, Oregon Health & Science University, Portland, Oregon, USA (co-PI, CHILD-BRIGHT); Dr Khush Amaria, CBT Associates of Toronto: Cognitive Behavioural Therapy Services, Toronto, Ontario, Canada; Dr Ronen Rozenblum, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA. Patient and Family Advisory Council: Donna Thomson, parent/family advisor in Ontario, Canada; Roger Stoddard, parent/family advisor in New Brunswick, Canada; JoAnne Mosel, parent/family advisor in Quebec, Canada; Connie Putterman, parent/family advisor in Ontario, Canada; Kinga Pozniak, parent/family advisor in Ontario, Canada; Nathan Tasker, young adult/patient Advisor in Ontario, Canada; Julia Hanes, young adult/patient advisor in Ontario, Canada; Kyle Chambers, young adult/patient advisor in Ontario, Canada; Jessica Havens, young adult/patient advisor in Alberta, Canada; Claire Dawe-McCord, young adult/patient advisor in Ontario, Canada; Dana Arafeh, young adult/patient advisor in Ontario, Canada; Randomised controlled trial (RCT) investigators: Dr Hana Alazem, University of Ottawa, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Dr John Andersen, University of Alberta, Glenrose Rehabilitation Hospital, Edmonton, Alberta, Canada; Dr Rima Azar, Mount Allison University, Sackville, New Brunswick, Canada; Dr Kerry Boyd, McMaster University, McMaster Children’s Hospital, Hamilton, Ontario, Canada; Dr Caitlin Cassidy, Western University, St Joseph's Health Care London, Ontario, Canada; Jamie Churchill, McMaster Children’s Hospital, Hamilton, Ontario, Canada; CJ Curran, Holland Bloorview Kids Rehabilitation Hospital, Toronto, Ontario, Canada; Dr Shelley Doucet, University of New Brunswick, Saint John, New Brunswick, Canada; Dr Anne Fournier, CHU Mère-Enfant, Sainte Justine Hospital, Montreal, Quebec, Canada; Dr Sarah Gander, Dalhousie University, Memorial University of Newfoundland, Saint John Regional Hospital, Saint John, New Brunswick, Canada; Dr Andrew Mackie, University of Alberta, Stollery Children's Hospital, Edmonton, Alberta, Canada; Dr Anna McCormick, University of Ottawa, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Dr Ronit Mesterman, McMaster University, McMaster Children’s Hospital, Hamilton, Ontario, Canada; Dr Maryam Oskoui, McGill University and Montreal Children's Hospital, McGill University Health Centre, Montreal, Quebec, Canada; Dr Janet Rennick, McGill University and Montreal Children’s Hospital, McGill University Health Centre, Montreal, Quebec, Canada; Dr Jordan Sheriko, Dalhousie University, IWK Rehabilitation Services, Halifax, Nova Scotia, Canada; Dr Kathy Speechley, Western University, London, Ontario, Canada; Dr Lehana Thabane, McMaster University, Hamilton, Ontario, Canada; Kelly Wynne, McMaster Children’s Hospital, Hamilton, Ontario, Canada; Dr Lonnie Zwaigenbaum, University of Alberta, Glenrose Rehabilitation Hospital, Edmonton, Alberta, Canada. RCT coordinators: Barb Galuppi, McMaster University, Hamilton, Ontario, Canada; Nadilein Mahlberg, McMaster University, Hamilton, Ontario, Canada; Sonya Strohm, McMaster University, Hamilton, Ontario, Canada; Alicia Via-Dufresne Ley, McGill University, Montreal, Quebec, Canada. RCT research assistants: Fabiola Breault, CHU Mère-Enfant, Sainte Justine Hospital, Montreal, Quebec, Canada; Yomna Elshamy, University of Alberta, Edmonton, Alberta, Canada; Rocio Gutierrez, CHU Mère-Enfant, Sainte Justine Hospital, Montreal, Quebec, Canada. Hashaam Hasan, Holland Bloorview Kids Rehabilitation Hospital, Toronto, Ontario, Canada; Rhiannon Hicks, London Health Sciences Centre, London, Ontario, Canada; Linda Nguyen, McMaster University, Hamilton, Ontario, Canada; André Pépin, Montreal Children’s Hospital, Montreal, Quebec, Canada; Rochelle Sorzano, London Health Sciences Centre, London, Ontario, Canada; Sarah Zaidi, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada; Dr Wendy J. Ungar, Child Health Evaluative Sciences, Hospital for Sick Children Research Institute; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada; Dr Jennifer D. Zwicker, School of Public Policy and Faculty of Kinesiology, University of Calgary, Calgary, AB, Canada; Dr Myla E. Moretti, Ontario Child Health Support Unit, Clinical Trials Unit, The Hospital for Sick Children; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada. Sponsor: McMaster University, 1280 Main Street West, Hamilton, Ontario, Canada L8S 4L8. (905) 525-9140.
Contributors: JWG and AM are the guarantors; JWG, AM, KA, RR, AK and LT contributed to the study conception and design; JWG, BG and LN contributed to the drafting of the manuscript; JWG, KA, AK, RR, LT, BG, LN, SS, NM, AVDL and AJM were involved in the critical revision; all the authors, Patient and Family Advisory Council members and randomised controlled trial investigators reviewed the manuscript and gave their input and final approval.
Formal analysis
Funding: We gratefully acknowledge funding from the Child Health Initiatives Limiting Disability–Brain Research Improving Growth and Health Trajectories network, funded under the Canadian Institutes of Health Research (CIHR-SCA-145104) Strategy for Patient-Oriented Research initiative, and with funding partner support from Montreal Children’s Hospital Foundation, Faculty of Health Sciences of McMaster University, New Brunswick Health Research Foundation, McMaster Children’s Hospital Foundation and Hamilton Health Sciences.
Competing interests: JWG and AJM received research grants from the Canadian Institutes of Health Research Strategy for Patient-Oriented Research. JWG holds the Scotiabank Chair in Child Health Research. AK and RR were paid in part for their work as consultants. BG, LN, SS, NM and AV-D-L were paid for their work as project staff members. LT was paid in part for his work as statistical consultant.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
CHILD-BRIGHT READYorNotTM Brain-Based Disabilities Trial Study Group:
Donna Thomson, Roger Stoddard, JoAnne Mosel, Connie Putterman, Kinga Pozniak, Nathan Tasker, Julia Hanes, Kyle Chambers, Jessica Havens, Claire Dawe-McCord, Dana Arafeh, Hana Alazem, John Andersen, Rima Azar, Kerry Boyd, Caitlin Cassidy, Jamie Churchill, CJ Curran, Shelley Doucet, Anne Fournier, Sarah Gander, Andrew Mackie, Anna McCormick, Ronit Mesterman, Maryam Oskoui, Janet Rennick, Jordan Sheriko, Kathy Speechley, Kelly Wynne, Lonnie Zwaigenbaum, Fabiola Breault, Yomna Elshamy, Rocio Gutierrez, Hashaam Hasan, Rhiannon Hicks, André Pépin, Rochelle Sorzano, Sarah Zaidi, Wendy J Ungar, Jennifer Zwicker, and Myla E Moretti
Ethics statements
Patient consent for publication
Not required.
References
- 1.Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children's hospitals. Pediatrics 2011;128:5–13. 10.1542/peds.2010-2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porterfield SL, DeRigne L. Medical home and out-of-pocket medical costs for children with special health care needs. Pediatrics 2011;128:892–900. 10.1542/peds.2010-1307 [DOI] [PubMed] [Google Scholar]
- 3.DuPaul GJ, Carson KM, Fu Q. Medical home care for children with special needs: access to services and family burden. Child Heal Care 2013;42:27–44. 10.1080/02739615.2013.753813 [DOI] [Google Scholar]
- 4.Miller JE, Nugent CN, Russell LB. Risk factors for family time burdens providing and arranging health care for children with special health care needs: lessons from nonproportional odds models. Soc Sci Res 2015;52:602–14. 10.1016/j.ssresearch.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics 2014;134:e1628–47. 10.1542/peds.2014-1956 [DOI] [PubMed] [Google Scholar]
- 6.Blum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for adolescent medicine. J Adolesc Health 1993;14:570–6. 10.1016/1054-139x(93)90143-d [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine . A consensus statement on health care transitions for young adults with special health care needs. Pediatrics 2002;110:1304–6. [PubMed] [Google Scholar]
- 8.Kaufman M, Pinzon J. Transition to adult care for youth with special health care needs. Paediatr Child Health 2007;12:785–8. 10.1093/pch/12.9.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prior M, McManus M, White P, et al. Measuring the "triple aim" in transition care: a systematic review. Pediatrics 2014;134:e1648–61. 10.1542/peds.2014-1704 [DOI] [PubMed] [Google Scholar]
- 10.Rozenblum R, Miller P, Pearson D. Patient-centered healthcare, patient engagement and health information technology: The perfect storm. In: Grandon M, Rozenblum R, Bates D, eds. Information technology for patient empowerment in healthcare. Berlin: Walter de Gruyter Inc, 2015: 3–22. [Google Scholar]
- 11.Acuña Mora M, Sparud-Lundin C, Bratt E-L, et al. Person-Centred transition programme to empower adolescents with congenital heart disease in the transition to adulthood: a study protocol for a hybrid randomised controlled trial (STEPSTONES project). BMJ Open 2017;7:e014593. 10.1136/bmjopen-2016-014593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creswell J, Plano Clark VL. Complex Applications of Core Mixed Methods Designs. In: Designing and conducting mixed methods research. Thousand Oaks, California: Sage Publications Inc, 2018: 101–41. [Google Scholar]
- 13.Klassen AF, Grant C, Barr R, et al. Development and validation of a generic scale for use in transition programmes to measure self-management skills in adolescents with chronic health conditions: the TRANSITION-Q. Child Care Health Dev 2015;41:547–58. 10.1111/cch.12207 [DOI] [PubMed] [Google Scholar]
- 14.Gorter JW, Klassen A, Grant C. Validation of the TRANSITION-Q across sites and conditions in Ontario. In: Inaugural children’s healthcare canada canadian transition to adulthood pop-up event. virtual, 2021. Available: https://www.childrenshealthcarecanada.ca/transitions-poster-hall/2021/1/11/validation-of-the-transition-q-across-sites-and-conditions-in-ontario
- 15.Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ--Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71. 10.1093/jpepsy/jsp128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackie AS, Islam S, Magill-Evans J, et al. Healthcare transition for youth with heart disease: a clinical trial. Heart 2014;100:1113–8. 10.1136/heartjnl-2014-305748 [DOI] [PubMed] [Google Scholar]
- 17.Parfeniuk S, Petrovic K, MacIsaac PL, et al. Transition readiness measures for adolescents and young adults with chronic health conditions: a systematic review. J Transit Med 2020;2:1–16. 10.1515/jtm-2020-0020 [DOI] [Google Scholar]
- 18.Dedding C, Cardol M, Eyssen ICJM, et al. Validity of the Canadian occupational performance measure: a client-centred outcome measurement. Clin Rehabil 2004;18:660–7. 10.1191/0269215504cr746oa [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. 10.1097/00005650-199902000-00003 [DOI] [PubMed] [Google Scholar]
- 20.King S, King G, Rosenbaum P. Evaluating health service delivery to children with chronic conditions and their families: development of a refined measure of processes of care (MPOC−20). Child Health Care 2004;33:35–57. 10.1207/s15326888chc3301_3 [DOI] [Google Scholar]
- 21.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horsman J, Furlong W, Feeny D, et al. The health Utilities index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1:54. 10.1186/1477-7525-1-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungar WJ, Tsiplova K, Millar N, et al. Development of the resource use questionnaire (RUQ–P) for families with preschool children with neurodevelopmental disorders: validation in children with autism spectrum disorder. Clin Pract Pediatr Psychol 2018;6:164–78. 10.1037/cpp0000226 [DOI] [Google Scholar]
- 24.Brooke J. Sus: a quick and dirty usability scale. Usability Eval Ind 1995;189. [Google Scholar]
- 25.Government of Canada . Coronavirus disease (COVID-19) [Internet], 2020. Available: https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19.html [Accessed 4 Jun 2020].
- 26.Public Safety Canada . Guidance on essential services and functions in Canada during the COVID-19 pandemic [Internet], 2020. Available: https://www.publicsafety.gc.ca/cnt/ntnl-scrt/crtcl-nfrstrctr/esf-sfe-en.aspx [Accessed 4 Jun 2020].
- 27.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archibald MM, Ambagtsheer RC, Casey MG, et al. Using Zoom videoconferencing for qualitative data collection: perceptions and experiences of researchers and participants. Int J Qual Methods 2019;18:160940691987459–18. 10.1177/1609406919874596 [DOI] [Google Scholar]
- 31.Gray LM, Wong-Wylie G, Cook K. Expanding qualitative research interviewing strategies: Zoom video communications. Qual Rep 2020;25:1292–301. [Google Scholar]
- 32.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med 1990;9:65–72. 10.1002/sim.4780090113 [DOI] [PubMed] [Google Scholar]
- 34.Brown H, Prescott R. Multi-centre trials and meta-analyses. In: Applied mixed models in medicine. 210. Chichester, UK: John Wiley & Sons, Ltd, 2014. [Google Scholar]
- 35.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 36.Alsem MW, Berkhout JJ, Buizer AI. Therapy needs and possibilities in paediatric rehabilitation during the COVID-19 lockdown in the Netherlands. Child Care Health Dev 2020;46:749–50. 10.1111/cch.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-048756supp001.pdf (860.4KB, pdf)
bmjopen-2021-048756supp002.pdf (153.4KB, pdf)
bmjopen-2021-048756supp003.pdf (258.4KB, pdf)
bmjopen-2021-048756supp004.pdf (71.8KB, pdf)
bmjopen-2021-048756supp005.pdf (27KB, pdf)
bmjopen-2021-048756supp006.pdf (69.2KB, pdf)