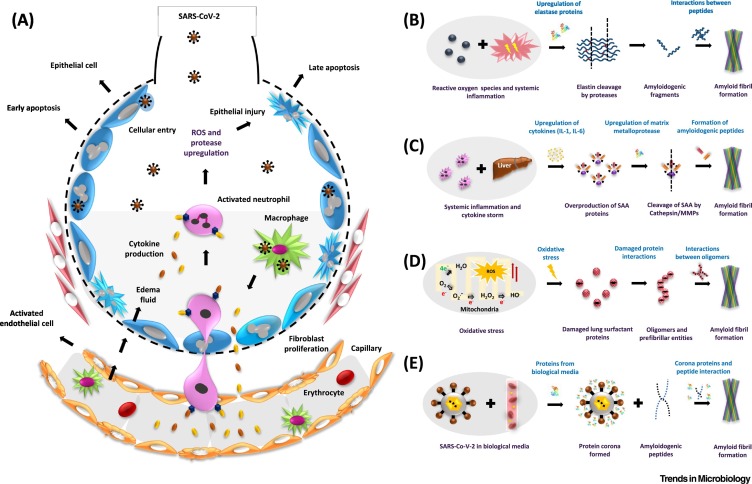
Figure 1.
The possible pathways of amyloid formation in severe acute respiratory syndrome coronavirus-2/coronavirus disease-2019 (SARS-CoV-2/COVID-19)-induced acute respiratory distress syndrome (ARDS).
(A) The pathophysiology of ARDS. The entry of SARS-CoV-2 inside the alveoli and engulfment of the viral particles by macrophages leads to the release of chemokines. These act as signaling molecules for circulating macrophages and neutrophils which then become activated. As a result, neutrophils upregulate the expression of cytokines and proteases that are responsible for inducing systemic inflammation and injury to epithelial and endothelial cells. The loss in permeability of endothelial cells allows entry of fluid into the alveoli, resulting in hypoxia and dyspnea. (B) The inflammatory response upregulates the enzyme elastase which might cleave elastin fibers to form amyloidogenic peptides. (C) Inflammation and subsequent upregulation of cytokines [interleukin (IL)-1, IL-6] also affects distant organs such as the liver and induces overproduction of serum amyloid A (SAA) proteins. SAA is cleaved by upregulated matrix metalloproteases (MMPs) and forms amyloid-forming peptides. (D) Oxidative stress, induced due to SARS-CoV-2 infection and ARDS, could lead to the production of reactive oxygen species (ROS); this perturbs redox homeostasis, resulting in misfolding and aggregation of metastable proteins such as pulmonary surfactant protein SP-C. (E) SARS-CoV-2 in the blood, or in bronchoalveolar lavage fluid, might form an extra protein corona layer around itself – which might induce amyloid formation.