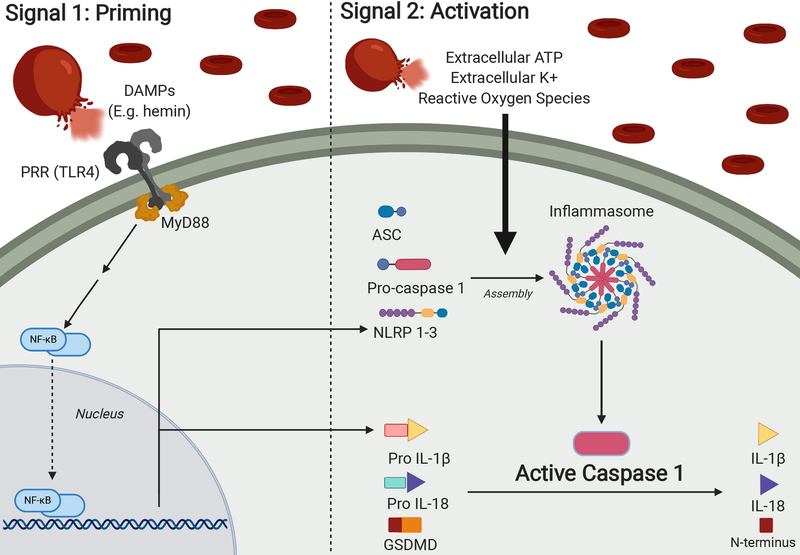
Figure 3.
The inflammasome complex and activation of caspase-1. Upon rupture of an intracranial aneurysm, blood rushes into the subarachnoid space causing increased intracranial pressure and reduced cerebral blood flow. As these extravasated red blood cells degrade, they release intracellular contents such as hemoglobin, hemin, bilirubin, potassium, adenosine triphosphate (ATP) and others.(48–50) In addition to driving formation of reactive oxygen species, these degraded blood products serve as damage-associated molecular patterns (DAMPs) that will drive a two-step process of priming and activation of the inflammasome complex.
In the priming step, DAMPs will be recognized by pattern recognition receptors (PRRs), which will signal to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) to upregulate DNA transcription of Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing (NLRPs) NOD-like-receptors and pro-IL-1β.(51–55) There are various subtypes of inflammasomes expressed by multiple cell types in the CNS, each characterized by differing protein constitution, with NLRP3 in microglia being the most well-known.(51) NLRPs and pro- IL-1β are necessary components for inflammasome and caspase-1 activity, making their upregulation by NF-κB an important priming step.
A second activation signal, mediated by DAMPs derived from extravasated blood, will drive NLRPs, adapter protein apoptosis associated speck like protein containing a caspase recruitment domain (ASC) and pro-caspase-1 to form the multimeric inflammasome complex. The inflammasome can lead to the activation of caspase-1 by cleaving pro-caspase-1 into caspase-1, the active form of the enzyme, which will then in turn cleave other proinflammatory molecules into their respective active forms. There are two main effects of these proteins – inflammation, mediated by interleukins IL-1β and IL-18, and pyroptosis, mediated by the cleaved N-terminus of gasdermin D. Pyroptosis is a form of cell death involving the rupture of cell membranes and release of proinflammatory intracellular contents, making it distinct from the non-inflammatory cell death observed with apoptosis.(56,57) Figure created with BioRender.com.