Fig. 3. Glucocorticoid treatment and stress promoted Ahi1 protein degradation through ubiquitination.
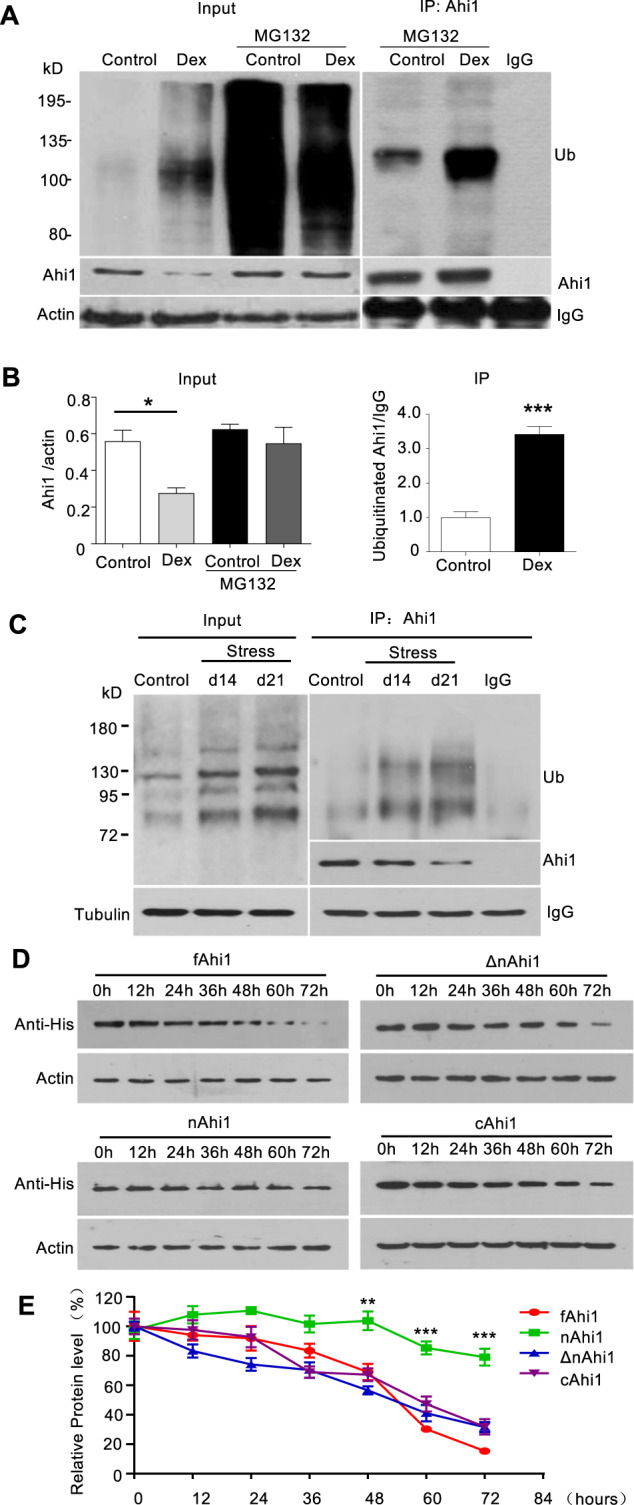
A PC12 cells were treated with or without Dex (20 μM) for 72 h, and then treated with or without MG132 (5 μM) for 10 h. Cell lysates were collected for the immunoprecipitation with Ahi1 antibody. Ahi1 and ubiquitinated proteins were detected with anti-Ahi1 and anti-ubiquitin antibodies. B The relative levels of Ahi1 (Ahi1/β-actin) (F(1,8) = 3.341, *P = 0.0175) and ubiquitinated Ahi1 (Ub/IgG) (t(4) = 8.521, ***P = 0.001) were obtained from three independent experiments. C After spatial restraint stress for 14 days or 21 days, mouse hypothalamic tissues were collected for the immunoprecipitation with Ahi1 antibody. Ahi1 and ubiquitinated Ahi1 protein were examined by western blotting. D Different Ahi1-His fusion proteins expressing full length (fAhi1, aa 1–1047), N-terminal (nAhi1, aa 1–284), Ahi1 without N-terminal region (ΔnAhi1, aa 285–1047), and C-terminal (cAhi1, aa 651–1047) were transfected into PC12 cells. The co-transfected cells were treated with Dex (20 μM) for different times (0–72 h). Ahi1-His levels were determined by Western blotting with His antibody. E The degradation of different Ahi1-His fusion proteins (F(3,84) = 55.50, ***P < 0.0001 versus control fAhi1, n = 4 cell samples).
