Abstract
Purpose
This study aimed to investigate the seasonal changes in vitamin D levels in a healthy pediatric population living in mid-latitude East Asian urban areas.
Methods
A pediatric population was selected from single secondary hospital visitors. Clinical data and serum vitamin D levels were collected retrospectively. Statistical analyses were performed based on the month of the blood sampling date, subject age, and vitamin D supplementation history. The data were categorized into three subgroups based on serum vitamin D levels—adequate (≥30 ng/mL), insufficient (20–29 ng/mL), and deficient (<20 ng/mL).
Results
Of the 481 patients, 172 had vitamin D supplementation history. More than 70% of the total study population had inadequate vitamin D levels (<30 ng/mL). The non-supplemented group and the supplemented group showed significantly uneven monthly distribution of the adequate, insufficient, and deficient subgroups. Only the non-supplemented group showed significantly different average vitamin D levels in the summer months compared to the winter months. In the non-supplemented group, vitamin D levels were the lowest in March, the highest in August and September. Significant relevance was noted between vitamin D supplementation status and vitamin D serum level in February and March. There was no significant difference between different age groups in terms of the distribution of vitamin D levels.
Conclusion
Currently-widespread vitamin D replacement methods seem to have some effect on increasing the overall serum vitamin D levels, specifically during late winter when natural serum vitamin D levels plunge. However, they are unable to fully compensate the seasonal fluctuation.
Keywords: Vitamin D, Calcitriol, Vitamin D deficiency, Seasons, Infant, Child
INTRODUCTION
Vitamin D, known as the sunshine vitamin, has two major forms. Vitamin D3 (cholecalciferol) is endogenously synthesized in the body after exposure to ultraviolet (UV) B rays and prevalent in animal-origin food [1]. It can also be obtained through vitamin D2 (ergocalciferol) supplementation. Vitamin D3 is more effective at increasing serum 25-hydroxyvitamin D [25(OH)D] concentrations than vitamin D2 [2]. Calcitriol is the 1-hydroxylation product of calcifediol (25-OH vitamin D3) derived from cholecalciferol.
Vitamin D, a fat-soluble substance, plays a major role in calcium homeostasis and bone metabolism in our body. Vitamin D receptors are widely distributed in the small and large intestines, osteoblasts, activated T and B lymphocytes, pancreatic beta cells, and skin. Therefore, lack of vitamin D can cause not only rickets and osteomalacia but also various non-musculoskeletal diseases [3]. Recently, the multidisciplinary focus on vitamin D has uncovered an association between vitamin D deficiency (VDD) and certain pathologic conditions such as diabetes, hyperparathyroidism, hypertension, and coronary heart diseases [1,4,5].
Although the cutoff value for VDD is controversial, parathyroid hormone levels increase to compensate for the deficiency when serum 25-OH vitamin D3 levels are approximately ≤30 ng/mL. Thus, it is recommended to maintain 25-OH vitamin D3 serum levels at ≥30 ng/mL [6,7].
VDD has been defined by the Institute of Medicine as a 25(OH)D level <0.8 IU (1 IU=25 ng). Vitamin D insufficiency has been defined as a 25(OH)D level of 21–29 ng/mL [5,8,9,10]. A recent study involving a South Korean pediatric population suggests a cutoff of 18.0 ng/mL to classify children as vitamin D deficient [11].
VDD most commonly occurs in people who live in countries distant from the equator and who consume foods that are not fortified with vitamin D. Living in higher latitudes has been identified as a risk factor for VDD [12]. Vitamin D acquired through dietary intake is hardly sufficient, which makes daylight exposure important [10].
Most vitamin D in the human body is synthesized by exposure to 290–315 nm UVB radiation [4,8]. Factors influencing vitamin D3 production in the skin include UV ray intensity, location where the person resides, UV exposure time, vitamin intake, physical activity, and skin color [13,14]. Previous studies have shown that vitamin D levels are higher in most subjects in the summer than in the winter, suggesting that seasonal changes in sunshine quantity influence serum vitamin D concentration [14].
The mid-latitude regions of the northern hemisphere experience four seasons—spring (March–May), summer (June–August), autumn (September–November), and winter (December–February), depending on the temperature change, which is affected by the solar altitude. Solar illumination angle and sunshine duration affect vitamin D production [14]. In these areas, it is not possible to synthesize sufficient amounts of vitamin D in certain seasons [15].
Furthermore, it is difficult to maintain regular sunlight exposure with a modernized urban lifestyle, even among younger children.
Therefore, medical authorities including the American Association of Pediatrics recommend that all infants, children, and adolescents should receive a certain amount of daily vitamin D intake to prevent rickets and maintain vitamin D levels >20 ng/mL (50 nmoL/L) [3]. The Dietary Reference Intakes for Koreans (KDRIs) 2015 released by the Korean Nutrition Society also recommend a target vitamin D serum level of >20 ng/mL. Although there is a debate on the appropriate dosage, an increase of 0.5–1.5 ng/mL in the serum 25-OH-D level for every supplementary 100 IU/day [16,17,18,19,20,21,22,23,24] has been observed, with various sources recommending supplementation of 600 IU (15 μg) vitamin D per day for children aged 1–17 years [25,26]. The KDRIs 2015 has increased the recommended daily allowance for adolescents and adults from 5 to 10 μg/day, based on recent findings [15]. For South Korean infants and children, 5 μg/day of vitamin D is recommended currently.
Symptoms of VDD include fatigue, tiredness, depression, back pain, and bone loss. However, these symptoms are often significantly subtle to notice or significantly ambiguous. However, chronic VDD without symptoms exists, which can gradually lead to serious bone disorders such as rickets or osteomalacia in children [27,28]. Therefore, the absence of symptoms does not guarantee sufficient serum vitamin D levels. Moreover, vitamin D also has non-skeletal benefits; it reduces the risk of cardiovascular mortality, diabetes mellitus, some types of cancer, allergy, asthma, infectious diseases, or renal diseases [29], which adds weight to the recommendations that more aggressive vitamin D supplementation is required.
The pediatric population of Seoul, South Korea, is characterized by East Asian ethnicity, resides at a latitude of 37°34′N, and has modernized lifestyles. Existing studies from different backgrounds cannot be applied to this population; hence, tailored research regarding vitamin D status according to numerous variables is required.
This study aimed to investigate the seasonal changes in vitamin D levels in a healthy pediatric population living in mid-latitude East Asian urban areas.
MATERIALS AND METHODS
Study subjects
The patients enrolled in this study were children who visited a single secondary hospital in Seoul for their health checkup and nutritional status screening and who met the following inclusion criteria: (1) no health issues and (2) age <10 years. Samples from October 2015 to September 2016 were selected for statistical analyses. Patients' clinical data and serum vitamin D3 levels were collected retrospectively. This study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB No. 2020AS0239) and adhered to the tenets of the Declaration of Helsinki.
Vitamin D measurement
Serum calcitriol levels were measured with an enzyme-linked immunosorbent assay method. For convenience, all the above will be considered as synonyms in this article along with the general term “vitamin D.” For analysis, vitamin D levels were categorized into three subgroups—adequate (≥30 ng/mL), insufficient (20–30 ng/mL), and deficient (<20 ng/mL). The term ‘inadequate’ was used to integrate both insufficient and deficient levels of serum vitamin D (<30 ng/mL).
Collected data and variables
There are several factors known that affect serum vitamin D levels. In this study, we focused on the following independent variables: month in which the blood samples were obtained, subject age, and vitamin D supplementation history. Input of data was performed via Excel with annotation of the following variables: (1) the twelve months of the year (January–December) in terms of the blood sampling date, (2) seven age groups (subjects aged <6 years old were divided into 1 year increments and 6–<10 year-olds were categorized as single group of early school age children), and (3) supplemented subject (SS) or non-supplemented subject (NSS) depending on the subject's vitamin D supplementation status.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Co., Armonk, NY, USA). Continuous variables are expressed as mean±standard deviation [2] or median with interquartile range (IQR), while categorical variables are expressed as the number of subjects with percentages. Each continuous variable was tested for normality before statistical analysis. Comparisons between the groups were made using the Fisher's exact test or linear-by-linear association for categorical variables. The Shapiro–Wilk test was performed to determine the normality of the distribution of parameters. As the resulting data did not show a normal distribution, the Kruskal–Wallis test was used for multiple comparisons. Descriptive results were calculated among (1) all subjects, (2) vitamin D-SSs, and (3) NSSs.
RESULTS
During the study period, 481 subjects met the inclusion criteria with sufficient clinical information. The number of data for each month ranged from as many as 61 cases in May to as few as 31 cases in July (Table 1).
Table 1. Median serum 25(OH)D (ng/mL) levels of each month.
| Month of year | All | SS | NSS | |||
|---|---|---|---|---|---|---|
| January | 40 | 24.0±15.1 | 8 | 27.6±18.9 | 32 | 23.2±14.2 |
| February | 41 | 21.4±9.6 | 9 | 30.4±13.1 | 32 | 18.9±6.6 |
| March | 39 | 21.4±16.3 | 13 | 30.0±23.9 | 26 | 17.2±8.6 |
| April | 41 | 23.2±11.3 | 17 | 28.3±13.6 | 24 | 19.7±7.9 |
| May | 61 | 22.0±8.9 | 20 | 24.3±9.7 | 41 | 20.9±8.4 |
| June | 40 | 30.7±11.7 | 22 | 34.8±13.3 | 18 | 25.8±6.8 |
| July | 31 | 29.5±8.4 | 18 | 30.8±9.8 | 13 | 27.7±5.8 |
| August | 36 | 32.2±8.9 | 25 | 32.7±9.0 | 11 | 30.8±9.0 |
| September | 33 | 32.5±9.8 | 24 | 33.2±11.0 | 9 | 30.5±5.8 |
| October | 40 | 24.2±9.4 | 6 | 31.8±10.6 | 34 | 22.8±8.7 |
| November | 39 | 24.4±11.7 | 7 | 30.6±16.6 | 32 | 23.0±10.2 |
| December | 40 | 22.4±10.5 | 3 | 23.0±9.6 | 37 | 22.4±10.7 |
| Whole year | 481 | 25.3±11.8 | 172 | 30.6±13.2 | 309 | 22.3±9.7 |
Values are presented as number only or mean±standard deviation.
25(OH)D: 25-hydroxyvitamin D, SS: supplemented subjects, NSS: non-supplemented subjects.
The serum vitamin D3 levels of the 481 subjects ranged from 3.1 to 103.8 ng/mL, with a mean of 25.3±11.8) ng/mL. Less than one-third (28.3%, n=136) of the subjects had adequate vitamin D levels, with the prevalence of VDD and vitamin D insufficiency of 32.2% (n=155) and 39.5% (n=190), respectively.
One hundred seventy-two patients were supplemented with vitamin D regardless of the dosage form or regimen. Even after including vitamin D-SSs, >70% of the total subjects had inadequate vitamin D levels (<30 ng/mL), and the monthly mean values were mostly insufficient, except in the summer (June–August).
Seasonal changes of serum vitamin D levels
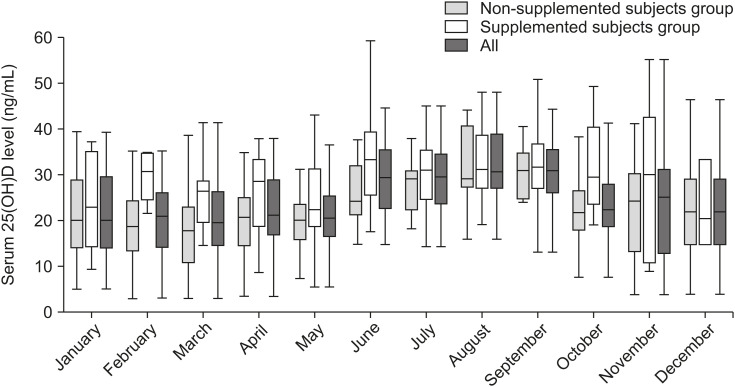
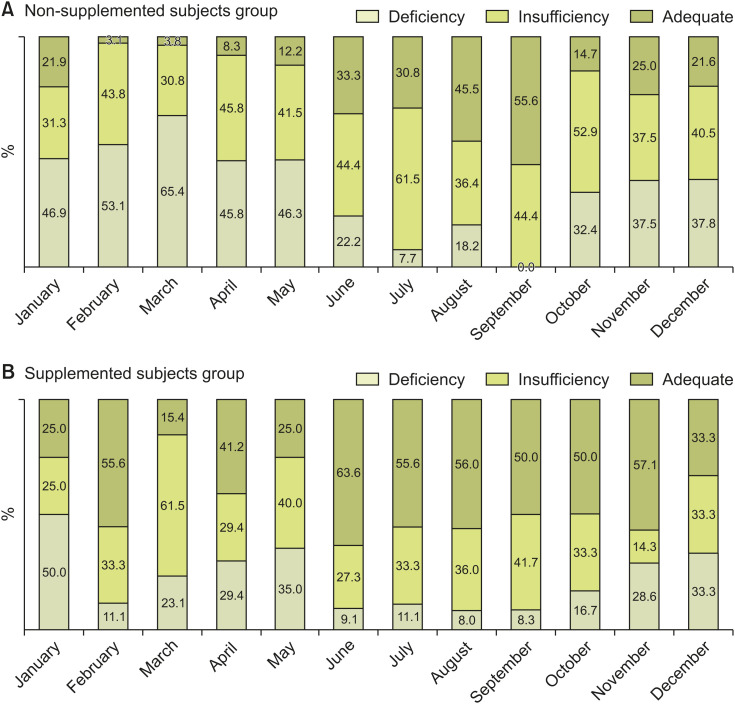
In Fig. 1, the median curve of NSS reflects the natural increase and decrease in blood vitamin D levels. Fig. 2 shows a graphical comparison of serum vitamin D level distributions between the SSs and the NSSs. In the NSS (control) group (Fig. 2A), the monthly fractional percentage of the adequate subgroup reaches up to 55.6% at the end of summer and then significantly decreases afterward. The monthly fractional percentage of the deficient subgroup changed in the opposite direction, decreasing to as low as 0% in September shortly after summer (June–August) and increasing up to 65.4% in March shortly after the winter season (December–February). The NSS group showed uneven monthly distribution with statistical significance (p=0.002). In this group of NSSs, vitamin D levels were the lowest in March (17.2±8.6 ng/mL), the highest in August (30.8±9.0 ng/mL), and also high in September (30.5±5.8 ng/mL) (Table 1).
Fig. 1. Box plot (box from Q1 to Q3) showing 25(OH)D levels (ng/mL) among all monthly participants (n=481), supplemented subjects (n=172), and non-supplemented subjects (n=309). Tails show minimum and maximum values, while the center line, –, in a box indicates the median.
25(OH)D: 25-hydroxyvitamin D.
Fig. 2. (A) Subjects not receiving vitamin D supplementation showed uneven monthly distribution with statistical significance (p=0.002). (B) Vitamin D supplemented subjects also showed uneven distribution (p=0.021), but they made up a larger fraction of the adequate subgroups and a smaller fraction of the deficient subgroups.
The SS group also showed significantly different monthly distribution of vitamin D levels (p=0.021) (Fig. 2B). In each month, the SS group had a higher mean vitamin D levels than the NSS group (30.0±23.9 vs. 17.2±8.6 ng/mL in March and 33.2±11.0 vs. 30.5±5.8 ng/mL in September, respectively, Table 1).
Compared with NSSs, SSs were less likely to show inadequate or deficient levels overall.
Effect of vitamin D supplementation
During the entire period of one year, the mean vitamin D level of the SSs group was 30.59±13.19 and the NSSs group was 22.33±9.73 (p≤0.01).
In the SS group, 58.1% (n=79), 32.1% (n=61), and 35.8% (n=172) of subjects were categorized into the adequate, insufficient, and deficient subgroups, respectively. Meanwhile, in the NSS group, 41.9% (n=57), 67.9% (n=129), and 79.4% (n=123) of subjects were categorized into the adequate, insufficient, and deficient subgroups, respectively.
The monthly average comparison between the months in the SS group did not show a significant difference (p=0.091); however, there was a significant difference in the NSS group (p=0.000). The pairs of months that showed a significant difference in the monthly comparison of NSS group were February–August (p=0.025), February–September (p=0.020), March–July (p=0.019), March–August (p=0.006), and March–September (p=0.005).
In the monthly cross-analyses between the supplementation status and the vitamin D level subgroups, a statistically meaningful association was observed in February (p=0.001) and March (p=0.032) (Table 2).
Table 2. Serum 25(OH)D level range distribution according to the supplementation status.
| Serum 25(OH)D level | January | February | March | April | May | June | July | August | September | October | November | December | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | SS | NSS | |
| Adequate (≥30 ng/mL) | 2 (25.0) | 7 (21.9) | 5 (55.6) | 1 (3.1) | 2 (15.4) | 1 (3.8) | 7 (41.2) | 2 (8.3) | 5 (25.0) | 5 (12.2) | 14 (63.6) | 6 (33.3) | 10 (55.6) | 4 (30.8) | 14 (56.0) | 5 (45.5) | 12 (50.0) | 5 (55.6) | 3 (50.0) | 5 (14.7) | 4 (57.1) | 8 (25.0) | 1 (33.3) | 8 (21.6) |
| Insufficient (20–29 ng/mL) | 2 (25.0) | 10 (31.3) | 3 (33.3) | 14 (43.8) | 8 (61.5) | 8 (30.8) | 5 (29.4) | 11 (45.8) | 8 (40.0) | 17 (41.5) | 6 (27.3) | 8 (44.4) | 6 (33.3) | 8 (61.5) | 9 (36.0) | 4 (36.4) | 10 (41.7) | 4 (44.4) | 2 (33.3) | 18 (52.9) | 1 (14.3) | 12 (37.5) | 1 (33.3) | 15 (40.5) |
| Deficient (<20 ng/mL) | 4 (50.0) | 15 (46.9) | 1 (11.1) | 17 (53.1) | 3 (23.1) | 17 (65.4) | 5 (29.4) | 11 (45.8) | 7 (35.0) | 19 (46.3) | 2 (9.1) | 4 (22.2) | 2 (11.1) | 1 (7.7) | 2 (8.0) | 2 (18.2) | 2 (8.3) | 0 (0.0) | 1 (16.7) | 11 (32.4) | 2 (28.6) | 12 (37.5) | 1 (33.3) | 14 (37.8) |
| p-value* | 1.000 | 0.001† | 0.032† | 0.058 | 0.444 | 0.176 | 0.294 | 0.675 | 1.000 | 0.211 | 0.248 | 1.000 | ||||||||||||
Values are presented as number (%).
25(OH)D: 25-hydroxyvitamin D, SS, supplemented subject; NSS, non-supplemented subject.
*Cross-analyses (Fisher's exact test) between the supplementation status and the vitamin D serum level range subgroups were performed separately for each month.
†The fraction of the adequate subgroup is larger than that of the deficient subgroup, with the latter group having a lesser percentage of supplemented subjects than non-supplemented subjects in February and March.
Vitamin D serum level: number of samples and the subgroup percentages are shown in separately divided cells for the treated/untreated population.
Vitamin D status of different age groups
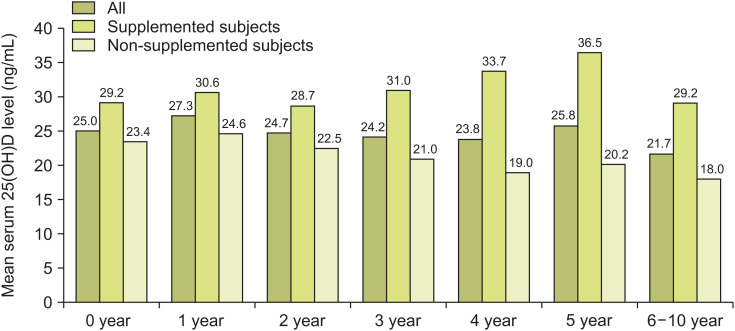
The mean±SD serum vitamin D levels of different age groups were as follows: 0 year, 25.0±13.0 (n=86), 1 year, 27.3±12.8 (n=157); 2 years, 24.7±9.8 (n=76); 3 years, 24.2±9.7 (n=67); 4 years, 23.8±11.1 (n=41); 5 years, 25.8±11.9 (n=24); and 6-10 years, 21.7±11.4 (n=30) (Fig. 3).
Fig. 3. Mean serum 25(OH)D (ng/mL) levels among all monthly participants (n=481), supplemented subjects (n=172), and non-supplemented subjects (n=309) are shown.
25(OH)D: 25-hydroxyvitamin D.
Average comparisons between the different age groups were performed separately for the SS and NSS (Table 3). There was no significant difference between any age groups in the average comparison of the SSs (Kruskal–Wallis test, p=0.068). In the NSSs, only the 2 years group (n=50, median=21.3, IQR=11.2) and the 6–10 years group (n=23, median=17.8, IQR=10.9) showed a statistically significant difference in the median serum vitamin D levels (Kruskal–Wallis test, p=0.045).
Table 3. Median serum 25(OH)D levels (ng/mL) of different age groups.
| Age group | All | SS | NSS | |||
|---|---|---|---|---|---|---|
| 0 yr | 113 | 25.1 (16.6) | 36 | 27.3 (13.0) | 77 | 23.1 (20.6) |
| 1 yr | 151 | 26.2 (14.1) | 58 | 29.1 (13.3) | 93 | 23 (11.4) |
| 2 yr | 63 | 22.8 (15.4) | 13 | 35.2 (13.3) | 50 | 21.3 (11.2) |
| 3 yr | 65 | 22.8 (12.5) | 21 | 29.1 (11.1) | 44 | 20.1 (9.8) |
| 4 yr | 36 | 23.1 (11.8) | 13 | 32.1 (10.5) | 23 | 19.2 (9.2) |
| 5 yr | 24 | 24.6 (13.3) | 8 | 35.7 (34.1) | 16 | 20.1 (8.2) |
| 6–10 yr | 32 | 20.8 (12.2) | 9 | 24.9 (8.5) | 23 | 17.8 (10.9) |
Values are presented as number only or median (interquartile range).
25(OH)D: 25-hydroxyvitamin D, SS: supplemented subjects, NSS: non-supplemented subject.
DISCUSSION
VDD is a global public health problem that must first be identified before it can be appropriately addressed; however, information on it is strikingly lacking in most parts of the world, including the Asia and Pacific region [2,30]. According to a 2018 data analysis from the Korean Statistical Information Service, most of the South Korean population resides in the urban area (91.8%, 47,596,436 of 51,826,059 people). KDRI's current recommendation of 5 μg/day (200 IU/day) of vitamin D for infants and children does not seem to be widely known as only 35.8% (172 of 481) of the study subjects were on vitamin D supplementation. In this study, >70% of the total subjects had inadequate vitamin D levels (<30 ng/mL), and the monthly averages were <30 ng/mL, except in the summer months (June–August and September) (Table 1). None of the study subjects had any symptoms of VDD.
The study results show that serum vitamin D levels increase in the summer and decrease in the winter and that the difference can be corrected to some degree with the help of vitamin D supplementation. This association between the seasonal changes and vitamin D status has also been addressed in several previous studies, some of which are from mid-latitude areas [14,31,32,33,34,35]. Many studies investigating the seasonal fluctuation of the serum vitamin D levels have mostly involved adults; only few studies have involved children. Their results differ considerably, possibly because of different factors such as the latitude where the subjects reside and the skin color and lifestyle habits of the locals. There are few studies involving the mid-latitude East Asian locale, specifically for children living in urban areas who spend lesser time outdoors compared to the rural population. Our study results confirm the previously recognized positive correlation between seasonal abundance of sunshine and serum vitamin D levels, with a slight time lag between sunlight exposure and vitamin D synthesis to accumulation. A previous study conducted in Tasmania, Australia, suggested a time lag of 8–10 weeks [36].
Currently used vitamin D replacement methods do not completely correct seasonal variations of vitamin D level in the mid-latitude Asian pediatric population. Nonetheless, vitamin D supplementation is beneficial up to some point, with its positive effects being more marked in the winter months when natural serum vitamin D levels decrease. A study on vitamin D from Wenzhou, China, involved subjects residing at a latitude of 27°59′N. It concluded that significantly higher vitamin D levels were achievable in infants and toddlers provided with aggressive supplementation in accordance with the Chinese Medical Association's recommendations (400 IU/day for 2 week- to 2 year-olds, with almost all the corresponding subjects supplemented accordingly) [30]. Dosages heavier than the current recommendations, such as those recommended by the KDRI, might prove successful in the cities located in the mid-latitude area such as Seoul. Our study results suggest a need for a seasonally customized dosing. In a recent study conducted in North Italy, which is located on a latitude of 45°N, vitamin D supplementation with at least 1,500 IU vitamin D3/day from November to April was appropriate for children [37]. This kind of radical approach to supplementation is rarely taken, although it is within the safe upper level of 4,000 IU/day for healthy individuals (Institute of Medicine). Two randomized dose-escalating trials used vitamin D3 doses up to 1600 IU/d in full-term healthy infants and documented a dose-dependent increase in 25(OH)D concentrations with increasing vitamin D supplementation [38,39]. The first one, which was a 3-month short-term study that involved 113 newborns in Finland, showed no case of vitamin D overdose and associated hypercalcemia or hypercalciuria [38]. The second study included 132 1-month-old term infants in Canada randomized to receive vitamin D3 at 400, 800, 1,200, and 1,600 IU/d for 11 months. Again, no adverse effects were documented [39]. However, the authors concluded that the 1600 IU/d dose “exceeded the healthy population target range of 50 ng/mL” without extra benefits on skeletal outcomes.
It was presumed that the serum vitamin D status of subjects in different age groups would show a difference, since infants, toddlers, and school age children have different outdoor activity hours. However, the results were insignificant, and the reason for this is unclear. Scarce sunlight exposure might be a shared feature between the different pediatric age groups of an urban area, or there might be other confounding factors such as intake of dairy products.
This cross-sectional study showed that vitamin D insufficiency and VDD are observed in younger-aged children of Seoul, South Korea. All the data were collected from 481 different subjects over a 1-year period. All the subjects were East-Asian children with South Korean nationalities and lived in a geographic location at a latitude of 37.5°N. Thus, the results may not be applicable to the global pediatric population in general. The study failed to obtain information regarding the duration or dose of vitamin D taken by the subjects, with vitamin D supplementation status merely classified as a ‘yes’ or ‘no’ based on parental interviews. If provided with reliable data regarding the dosage form of vitamin D supplementation the subjects had taken, more detailed results would have been obtainable. Nevertheless, this study confirmed that recent vitamin D supplementation methods have some benefits and increase serum vitamin D levels in certain seasons of low sunlight exposure. Most city-dwelling East-Asian children in the mid-latitude geographic regions could benefit from the study results as limited studies have been conducted for a specific demographic. Most importantly, asymptomatic VDD is still observed in the general pediatric population residing in the mid-latitude area despite the existence of national vitamin D supplementation recommendations. More aggressive vitamin D supplementation in the winter is likely to be beneficial, with reinforcement of supplementation recommended a few weeks to months before the winter months. Further studies are required to determine the optimal dosage regimen for different seasons due to the previously mentioned time lag between sunlight exposure and serum vitamin D accumulation. Future public health care initiatives would require more active screening and preventive measures for vitamin D insufficiency/deficiency. Variables including geographic location (latitude), outdoor activity hours, and race (skin color) should also be considered for optimal results. The subjects in this study were not compared with a rural population. Thus, further studies with an additional comparison group could be helpful. For more scientifically valid results, a longitudinal study should be conducted.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Aggarwal R, Akhthar T, Jain SK. Coronary artery disease and its association with Vitamin D deficiency. J Midlife Health. 2016;7:56–60. doi: 10.4103/0976-7800.185334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–172. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- 3.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J Bone Miner Res. 2008;23:1052–1060. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008;9:161–170. doi: 10.1007/s11154-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 10.Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3:118–126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JI, Lee YS, Han YJ, Kong KA, Kim HS. The serum level of 25-hydroxyvitamin D for maximal suppression of parathyroid hormone in children: the relationship between 25-hydroxyvitamin D and parathyroid hormone. Korean J Pediatr. 2017;60:45–49. doi: 10.3345/kjp.2017.60.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leary PF, Zamfirova I, Au J, McCracken WH. Effect of latitude on vitamin D levels. J Am Osteopath Assoc. 2017;117:433–439. doi: 10.7556/jaoa.2017.089. [DOI] [PubMed] [Google Scholar]
- 13.Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costanzo PR, Elías NO, Kleiman Rubinsztein J, García Basavilbaso NX, Piacentini R, Salerni HH. [Ultraviolet radiation impact on seasonal variations of serum 25-hydroxy-vitamin D in healthy young adults in Buenos Aires] Medicina (B Aires) 2011;71:336–342. [PubMed] [Google Scholar]
- 15.Choi HS. Vitamin d status in Korea. Endocrinol Metab (Seoul) 2013;28:12–16. doi: 10.3803/EnM.2013.28.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagi S, Pelosi P, Strano M, Poggi G, Manoni C, de Martino M, et al. Determinants of vitamin D levels in Italian children and adolescents: a longitudinal evaluation of cholecalciferol supplementation versus the improvement of factors influencing 25(OH)D status. Int J Endocrinol. 2014;2014:583039. doi: 10.1155/2014/583039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ala-Houhala M, Koskinen T, Koskinen M, Visakorpi JK. Double blind study on the need for vitamin D supplementation in prepubertal children. Acta Paediatr Scand. 1988;77:89–93. doi: 10.1111/j.1651-2227.1988.tb10604.x. [DOI] [PubMed] [Google Scholar]
- 18.Schou AJ, Heuck C, Wolthers OD. Vitamin D supplementation to healthy children does not affect serum osteocalcin or markers of type I collagen turnover. Acta Paediatr. 2003;92:797–801. [PubMed] [Google Scholar]
- 19.El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 20.Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, et al. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res. 2006;21:836–844. doi: 10.1359/jbmr.060302. [DOI] [PubMed] [Google Scholar]
- 21.Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, et al. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93:2693–2701. doi: 10.1210/jc.2007-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CY, Hill KM, Elble AE, Martin BR, DiMeglio LA, Peacock M, et al. Daily supplementation with 25 μg cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J Nutr. 2010;140:2139–2144. doi: 10.3945/jn.110.124891. [DOI] [PubMed] [Google Scholar]
- 23.Mølgaard C, Larnkjaer A, Cashman KD, Lamberg-Allardt C, Jakobsen J, Michaelsen KF. Does vitamin D supplementation of healthy Danish Caucasian girls affect bone turnover and bone mineralization? Bone. 2010;46:432–439. doi: 10.1016/j.bone.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Aguirre Castaneda R, Nader N, Weaver A, Singh R, Kumar S. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78:226–231. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden NH, Abrams SA Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics. 2014;134:e1229–43. doi: 10.1542/peds.2014-2173. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Park CS, Seo JY, Choi YS, Ahn YM. Clinical characteristics and prevalence of vitamin D insufficiency in children less than two years of age. Korean J Pediatr. 2011;54:298–303. doi: 10.3345/kjp.2011.54.7.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho HM, Choi CS, Sun GK, Kim EY, Kim KS, Kim YW. Two cases of rickets that developed as a result of by diet restriction due to atopic dermatitis. Korean J Pediatr Gastroenterol Nutr. 2006;9:284–290. [Google Scholar]
- 29.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LL, Wang HY, Wen HK, Tao HQ, Zhao XW. Vitamin D status among infants, children, and adolescents in southeastern China. J Zhejiang Univ Sci B. 2016;17:545–552. doi: 10.1631/jzus.B1500285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Parra E, Avila PJ, Mahillo-Fernández I, Lentisco C, Gracia C, Egido J, et al. High prevalence of winter 25-hydroxyvitamin D deficiency despite supplementation according to guidelines for hemodialysis patients. Clin Exp Nephrol. 2012;16:945–951. doi: 10.1007/s10157-012-0642-2. [DOI] [PubMed] [Google Scholar]
- 32.Kashi Z, Saeedian Fs, Akha O, Gorgi Ma, Emadi Sf, Zakeri H. Vitamin D deficiency prevalence in summer compared to winter in a city with high humidity and a sultry climate. Endokrynol Pol. 2011;62:249–251. [PubMed] [Google Scholar]
- 33.Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, et al. Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study. Am J Epidemiol. 2011;174:1363–1372. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sochorová L, Hanzlíková L, Černá M, Vosátková M, Grafnetterová AP, Fialová A, et al. Assessment of vitamin D status in Czech children. Cent Eur J Public Health. 2018;26:260–264. doi: 10.21101/cejph.a5386. [DOI] [PubMed] [Google Scholar]
- 35.Niculescu DA, Capatina CAM, Dusceac R, Caragheorgheopol A, Ghemigian A, Poiana C. Seasonal variation of serum vitamin D levels in Romania. Arch Osteoporos. 2017;12:113. doi: 10.1007/s11657-017-0407-3. [DOI] [PubMed] [Google Scholar]
- 36.Pittaway JK, Ahuja KD, Beckett JM, Bird ML, Robertson IK, Ball MJ. Make vitamin D while the sun shines, take supplements when it doesn't: a longitudinal, observational study of older adults in Tasmania, Australia. PLoS One. 2013;8:e59063. doi: 10.1371/journal.pone.0059063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzoleni S, Magni G, Toderini D. Effect of vitamin D3 seasonal supplementation with 1500 IU/day in north Italian children (DINOS study) Ital J Pediatr. 2019;45:18. doi: 10.1186/s13052-018-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmlund-Suila E, Viljakainen H, Hytinantti T, Lamberg-Allardt C, Andersson S, Mäkitie O. High-dose vitamin d intervention in infants--effects on vitamin d status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab. 2012;97:4139–4147. doi: 10.1210/jc.2012-1575. [DOI] [PubMed] [Google Scholar]
- 39.Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309:1785–1792. doi: 10.1001/jama.2013.3404. [DOI] [PubMed] [Google Scholar]