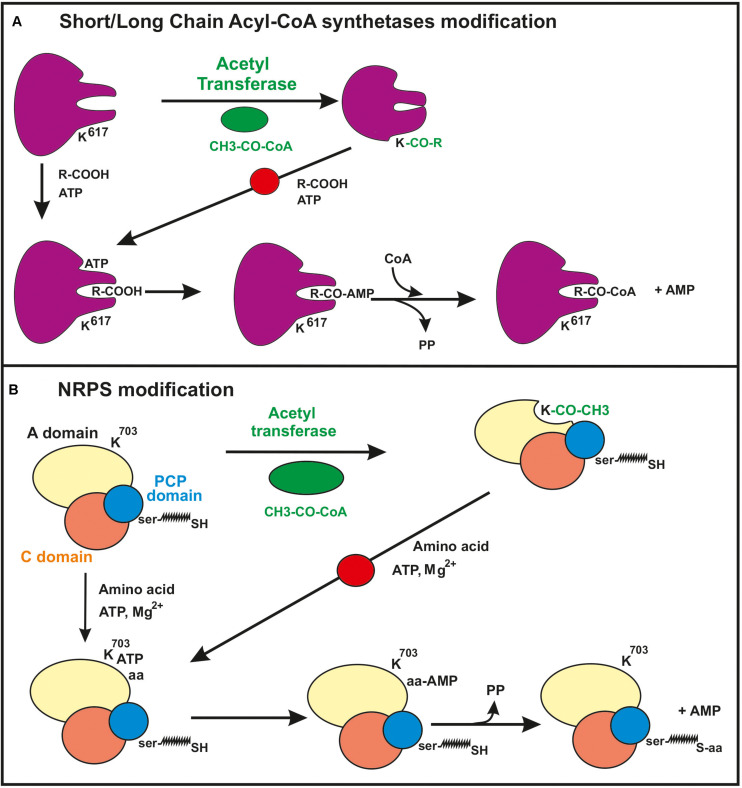
FIGURE 1.
Mechanism of inhibition by acetylation of the first step of the activation reaction of short/long chain fatty acid in acyl-CoA synthetases [panel (A) and amino acids in non-ribosomal peptide synthetases (NRPSs) (panel B)]. (A) In the short/long chain acyl-CoA synthetases a lysine residue (K617) in the acetoacetyl-CoA synthetase protein is acetylated resulting in inhibition of the formation of the acyl-AMP intermediate. (B) Inhibition by lysine acetylation of the formation of the aminoacyl-AMP intermediate of NRPSs. The lysine residue in the studied NRPS of S. roseosporus corresponds to K703. This acetylation prevents the formation of the aminoacyl-AMP (see text for details). The domains A (for amino acid activation), PCP (peptidyl carrier protein) with the phosphopantetheinyl arm and C (condensation domain) of the NRPS are shown. Note the similarity of the molecular mechanisms involved in the modification of acyl-CoA synthetase and NRPSs. The red circle on the arrow indicated that this reaction is blocked by the acylation of the enzyme.